
Biocytogen Enters into Antibody Agreement with Merck
BEIJING, CHINA, [April 29, 2022] – Biocytogen Pharmaceuticals (Beijing) Co., Ltd. (Biocytogen) announced that it has entered into an evaluation and option agreement with Merck…
Read more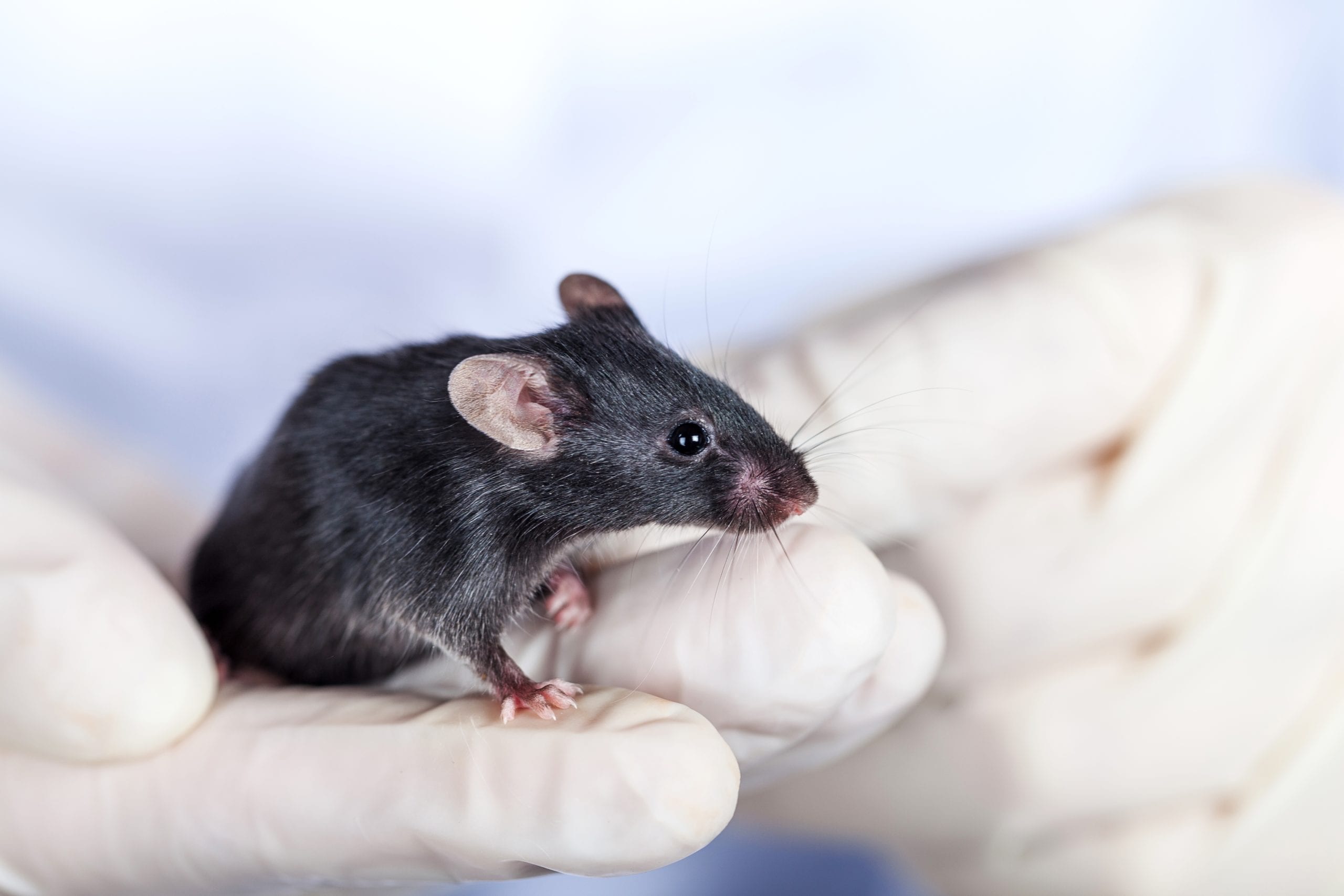
Biocytogen Signs RenMab™/RenLite® Licensing Agreement with BeiGene
Biocytogen Pharmaceuticals (Beijing) Co., Ltd. ("Biocytogen") reached an agreement with BeiGene (Beijing) Co., Ltd. ("BeiGene") (NASDAQ: BGNE; HKEX: 06160; SSE: 688235) for licensing Biocytogen’s fully…
Read more
Biocytogen Enters Agreement With CtM Bio to Co-develop TCR-Mimic Antibody-Based Multi-Specific T cell Engagers
BEIJING and SHANGHAI, April 18, 2022/PRNewswire/--Biocytogen Pharmaceuticals (Beijing) Co., Ltd. (hereafter referred to as "Biocytogen") announced a collaboration agreement with CtM Biotech (Shanghai) Co., Ltd…
Read more
Biocytogen Subsidiary Eucure Biopharma Completes First Patient Dosing for Phase I Clinical Trial of YH003 (Anti-CD40 mAb) Triple Combination Therapy
BEIJING, April 6, 2022 - Biocytogen subsidiary Eucure Biopharma announced the first patient dosing for a phase I clinical trial (No. YH003005) of YH003 (anti-CD40…
Read more
Biocytogen Expands Global Footprint with the Opening of Biocytogen Europe Innovation Center in Germany
Beijing, China and Heidelberg, Germany, March 28, 2022 - Biocytogen Pharmaceuticals (Beijing) Co., Ltd. (hereafter referred to as “Biocytogen”) announced the opening of the Biocytogen…
Read more
Biocytogen Subsidiary, Eucure Biopharma, Announces the First Patient Dosing for a Phase I Multi-regional Clinical Trial of YH002 (Anti-OX40 mAb) in Combination with YH001 (Anti-CTLA-4 mAb) in Australia
BEIJING, CHINA, March 8, 2022 - Eucure Biopharma, a wholly owned subsidiary of Biocytogen, announced the first patient dosing for a phase I multi-regional clinical trial…
Read more
Webinar: Mouse Models to Investigate New Treatments for Inflammatory Disease
We’re pleased to continue our new webinar series informing and updating our network of research professionals. We invite you to join us to learn about…
Read more
Biocytogen/Eucure Biopharma Announce the Completion of First Patient Dosing for Phase II Clinical Trial of YH003 in Australia
Beijing, China, December 9, 2021/PRNewswire/ – Eucure Biopharma, a wholly owned subsidiary of Biocytogen, announced the first patient dosing for a phase II clinical trial…
Read more
Biocytogen/Eucure Biopharma Announce the Completion of First Patient Dosing for Phase I Clinical Trial of YH004 (Anti-4-1BB Monoclonal Antibody) in Australia
BEIJING, CHINA, December 7, 2021 - Eucure Biopharma, a wholly owned subsidiary of Biocytogen, announced the first patient dosing for a phase I clinical trial of…
Read more
Biocytogen/Eucure Biopharma’s YH001 (Anti-CTLA-4 Monoclonal Antibody) Approved for Phase II Multi-Regional Clinical Trial by China National Medical Products Administration
BEIJING, November 9, 2021 - Eucure Biopharma, a wholly owned subsidiary of Biocytogen dedicated to developing proprietary antibody drugs, announced that the China National Medical Products Administration…
Read more

