Basic Information
-
Gene targeting strategy

-
Gene targeting strategy for B-hIL12A/hIL12B/hIL12RB1/hIL12RB2 mice.
The exons 1-7 of mouse Il12a gene that encode the full length coding sequence were replaced by human IL12A exons 1-7 in B-hIL12A/hIL12B/hIL12RB1/hIL12RB2 mice.
The exons 2-8 of mouse Il12b gene that encode the full length coding sequence and 3’UTR were replaced by human IL12B exons 2-8 in B-hIL12A/hIL12B/hIL12RB1/hIL12RB2 mice.
The extracellular and transmembrane region coding sequences of human IL12RB1 gene plus the mouse Il12rb1 cytoplasmic coding sequences were inserted into the exon1 of B-hIL12A/hIL12B/hIL12RB1/hIL12RB2 mice.
The extracellular region coding sequences of human IL12RB2 gene plus the mouse Il12rb2 transmembrane and cytoplasmic coding sequences were inserted into the exon2 of B-hIL12A/hIL12B/hIL12RB1/hIL12RB2 mice.
-
mRNA expression analysis

-
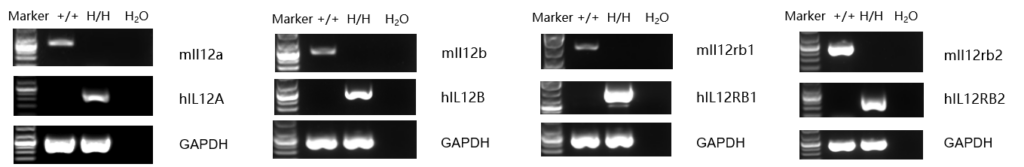
Strain specific analysis of IL12A, IL12B, IL12RB1 and IL12RB2 gene expression in B-hIL12A/hIL12B/hIL12RB1/hIL12RB2 mice by RT-PCR. Mouse Il12a was detectable in splenocytes of wild-type mice (+/+). Human IL12A was detectable only in homozygous B-hIL12A/hIL12B/hIL12RB1/hIL12RB2 mice but not in wild-type mice. Mouse Il12b, Il12rb1 and Il12rb2 were detectable in thymocytes of wild-type mice (+/+). Human IL12B, IL12RB1 and IL12RB2 were only detectable in homozygous B-hIL12A/hIL12B/hIL12RB1/hIL12RB2 mice but not in wild-type mice.
-
Protein expression analysis

-

Strain specific IL12p70 (IL-12A (p35) and IL-12B (p40) active heterodimer referred to as ‘p70’) expression analysis in homozygous B-hIL12A/hIL12B/hIL12RB1/hIL12RB2 mice by ELISA.
Serum was collected in wild-type (+/+) and homozygous B-hIL12A/hIL12B/hIL12RB1/hIL12RB2 mice (H/H) stimulated with anti-mCD3ε and anti-mCD28 in vivo (n=3), and analyzed by ELISA with species-specific IL12 ELISA kit. Mouse IL12p70 was detectable in wild-type mice. Human IL12p70 was exclusively detectable in homozygous B-hIL12A/hIL12B/hIL12RB1/hIL12RB2 mice but not in wild-type mice. ND: Not detectable.
-
Functional analysis

-
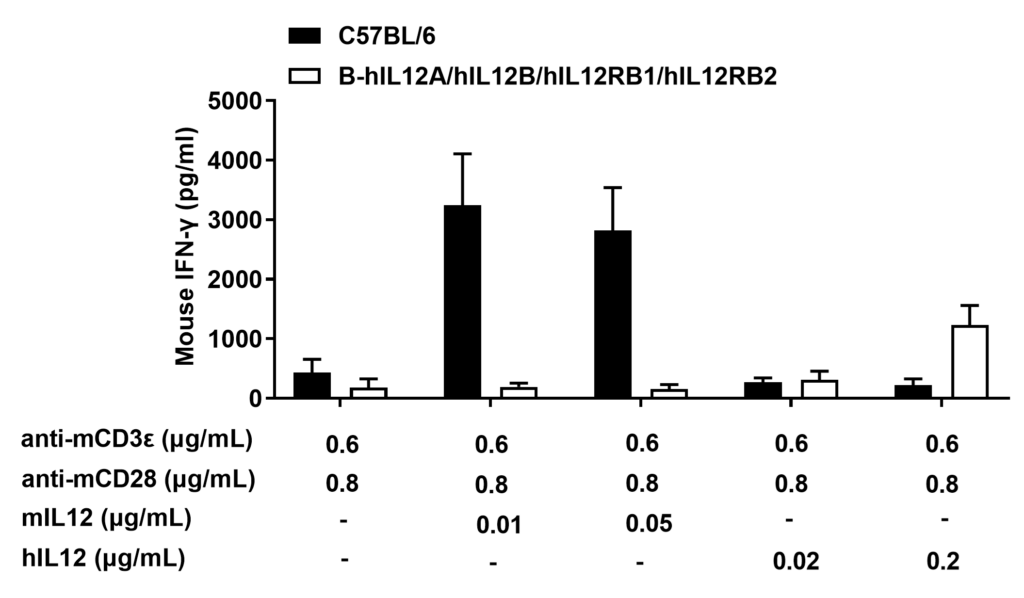
IL12 induced the IFN-γ production in CD4+ T cells sorted from splenocytes.
CD4+ T cells were sorted from the splenocytes in the wild-type (+/+) and homozygous B-hIL12A/hIL12B/hIL12RB1/hIL12RB2 mice (H/H) (n=3), the production of IFN-γ in supernatants were assessed after 48 h of incubation with 0.02 or 0.2 μg/mL rhIL-12 in combination with bead-associated CD3 and CD28 mAbs, under the condition in the panel. For comparison, with 0.01 or 0.05 μg/mL of rmIL-12 as control. Mouse IFN-γ were both increased after responsiveness to mIL-12 in wild-type mice and hIL-12 in homozygous B-hIL12A/hIL12B/hIL12RB1/hIL12RB2 mice (H/H). The humanized mice were successfully constructed.
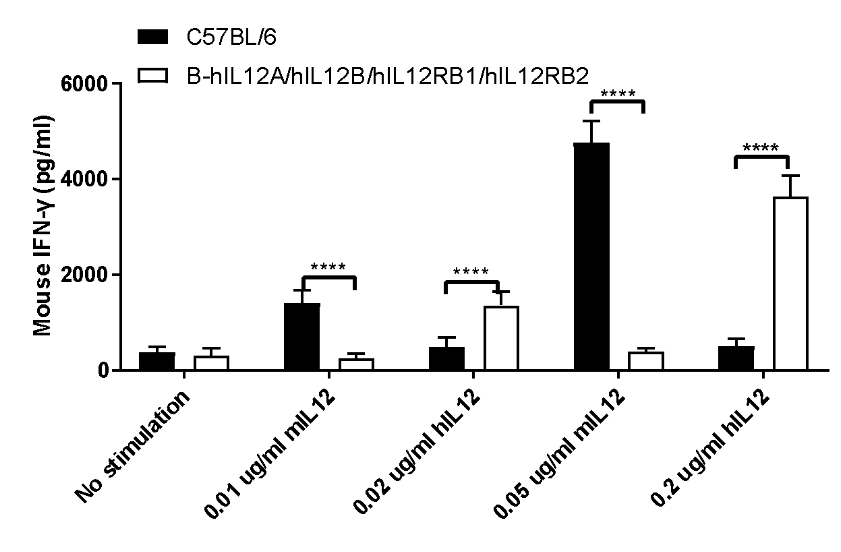
IL12 induced the IFN-γ production in CD4+ T cells sorted from splenocytes.
CD4+ T cells were sorted from the splenocytes in the wild-type (+/+) and homozygous B-hIL12A/hIL12B/hIL12RB1/hIL12RB2 mice (H/H) (n=3), the production of IFN-γ in supernatants were assessed after 48 h of incubation with 0.02 or 0.2 μg/mL rhIL-12 in combination with bead-associated CD3 (0.8 ug/mL) and CD28 (1.6 ug/mL) mAbs, under the condition in the panel. For comparison, with 0.01 or 0.05 μg/mL of rmIL-12 as control. Mouse IFN-γ were both increased after responsiveness to mIL-12 in wild-type mice and hIL-12 in homozygous B-hIL12A/hIL12B/hIL12RB1/hIL12RB2 mice (H/H). The humanized mice were successfully constructed.
-
Analysis of leukocytes cell subpopulation in spleen

-
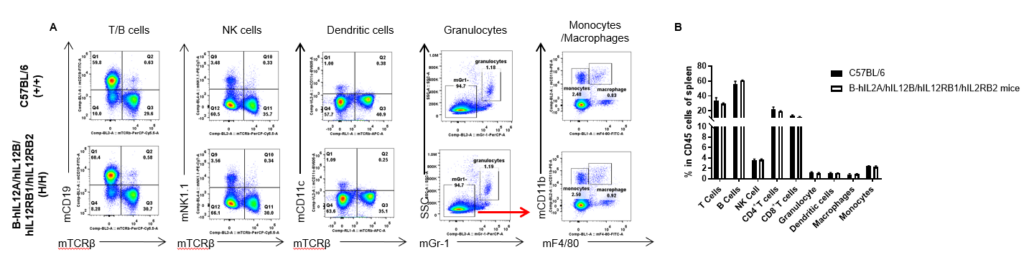
Analysis of spleen leukocyte subpopulations by FACS. Splenocytes were isolated from female C57BL/6 and B-hIL12A/hIL12B/hIL12RB1/hIL12RB2 mice (n=3, 6-week-old). Flow cytometry analysis of the splenocytes was performed to assess leukocyte subpopulations. A. Representative FACS plots. Single live cells were gated for the CD45+ population and used for further analysis as indicated here. B. Results of FACS analysis. Percent of T cells, B cells, NK cells, dendritic cells, granulocytes, monocytes and macrophages in homozygous B-hIL12A/hIL12B/hIL12RB1/hIL12RB2 mice were similar to those in the C57BL/6 mice, demonstrating that the humanization does not change the overall development, differentiation or distribution of these cell types in spleen. Values are expressed as mean ± SEM.
-
Analysis of T cell subpopulation in spleen

-
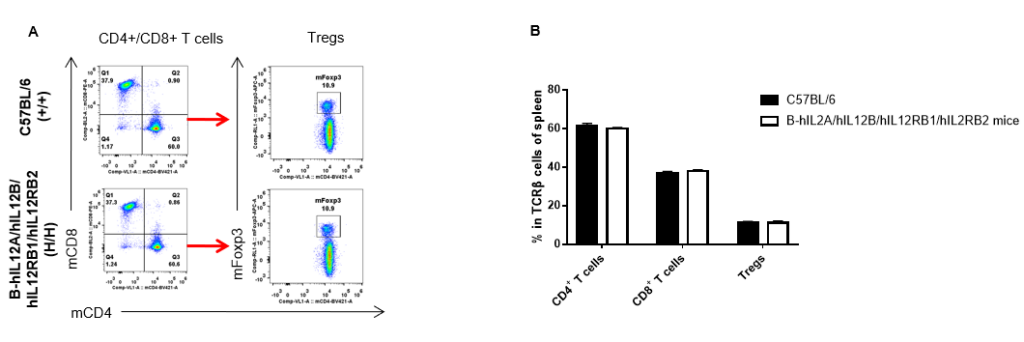
Analysis of spleen T cell subpopulations by FACS. Splenocytes were isolated from female C57BL/6 and B-hIL12A/hIL12B/hIL12RB1/hIL12RB2 mice (n=3, 6-week-old). Flow cytometry analysis of the splenocytes was performed to assess leukocyte subpopulations. A. Representative FACS plots. Single live CD45+ cells were gated for TCRβ+ T cell population and used for further analysis as indicated here. B. Results of FACS analysis. The percent of CD4+ T cells, CD8+ T cells and Tregs in homozygous B-hIL12A/hIL12B/hIL12RB1/hIL12RB2 mice were similar to those in the C57BL/6 mice, demonstrating that introduction of hIL12A/hIL12B/hIL12RB1/hIL12RB2 in place of its mouse counterpart does not change the overall development, differentiation or distribution of these T cell subtypes in spleen. Values are expressed as mean ± SEM.
-
Analysis of leukocytes cell subpopulation in lymph node

-
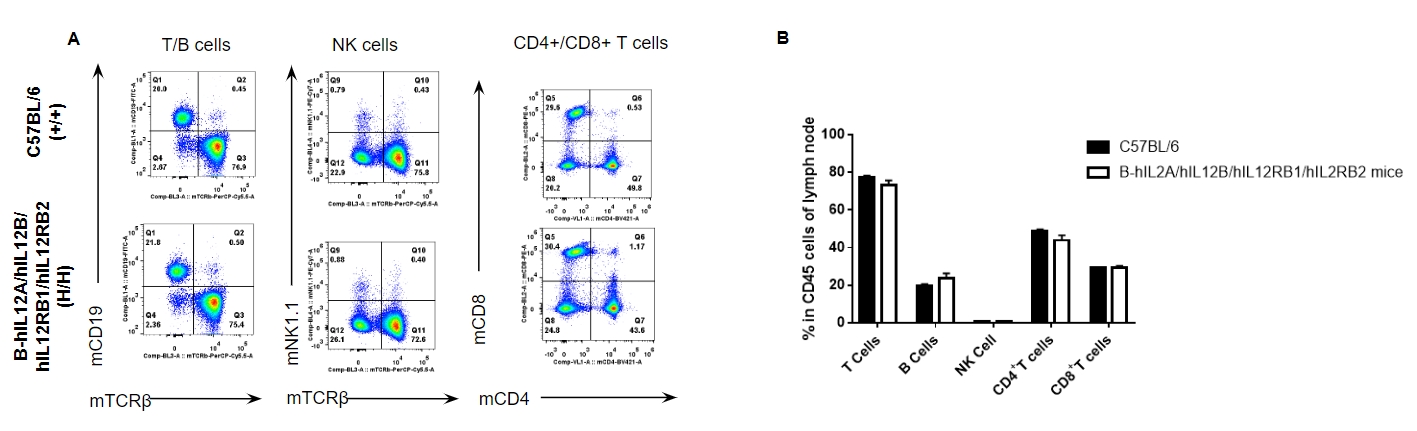
Analysis of lymph node leukocyte subpopulations by FACS. Lymph nodes were isolated from female C57BL/6 and B-hIL12A/hIL12B/hIL12RB1/hIL12RB2 mice (n=3, 6-week-old). Flow cytometry analysis of the lymph node was performed to assess leukocyte subpopulations. A. Representative FACS plots. Single live cells were gated for the CD45+ population and used for further analysis as indicated here. B. Results of FACS analysis. Percent of T cells, B cells, and NK cells in homozygous B-hIL12A/hIL12B/hIL12RB1/hIL12RB2 mice were similar to those in the C57BL/6 mice, demonstrating that the humanization does not change the overall development, differentiation or distribution of these cell types in lymph node. Values are expressed as mean ± SEM.
-
Analysis of T cell subpopulation in lymph node

-
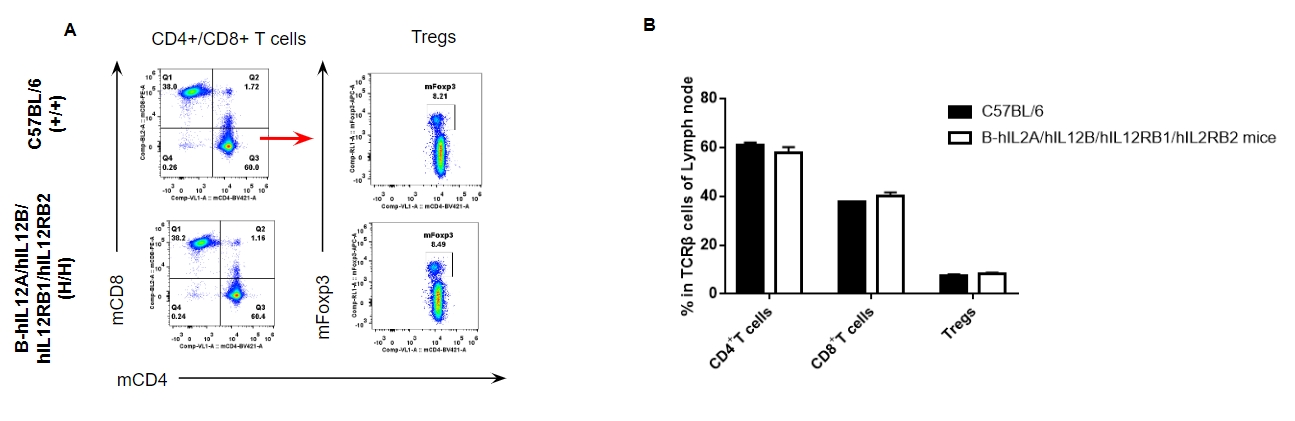
Analysis of lymph node T cell subpopulations by FACS. Lymph nodes were isolated from female C57BL/6 and B-hIL12A/hIL12B/hIL12RB1/hIL12RB2 mice (n=3, 6-week-old). Flow cytometry analysis of the lymph nodes was performed to assess leukocyte subpopulations. A. Representative FACS plots. Single live CD45+ cells were gated for TCRβ+ T cell population and used for further analysis as indicated here. B. Results of FACS analysis. The percent of CD4+ T cells, CD8+ T cells and Tregs in homozygous B-hIL12A/hIL12B/hIL12RB1/hIL12RB2 mice were similar to those in the C57BL/6 mice, demonstrating that introduction of hIL12A/hIL12B/hIL12RB1/hIL12RB2 in place of its mouse counterpart does not change the overall development, differentiation or distribution of these T cell subtypes in lymph nodes. Values are expressed as mean ± SEM.
-
Analysis of leukocytes cell subpopulation in blood

-
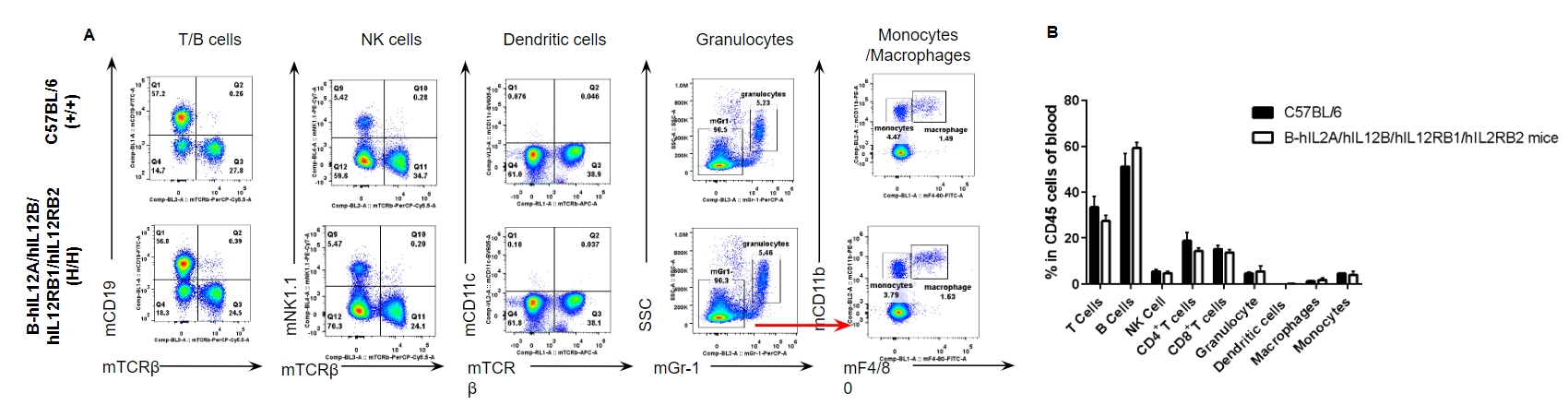
Analysis of blood leukocyte subpopulations by FACS. Blood were isolated from female C57BL/6 and B-hIL12A/hIL12B/hIL12RB1/hIL12RB2 mice (n=3, 6-week-old). Flow cytometry analysis of the blood was performed to assess leukocyte subpopulations. A. Representative FACS plots. Single live cells were gated for the CD45+ population and used for further analysis as indicated here. B. Results of FACS analysis. Percent of T cells, B cells, NK cells, dendritic cells, granulocytes, monocytes and macrophages in homozygous B-hIL12A/hIL12B/hIL12RB1/hIL12RB2 mice were similar to those in the C57BL/6 mice, demonstrating that the humanization does not change the overall development, differentiation or distribution of these cell types in blood. Values are expressed as mean ± SEM.
-
Analysis of T cell subpopulation in blood

-
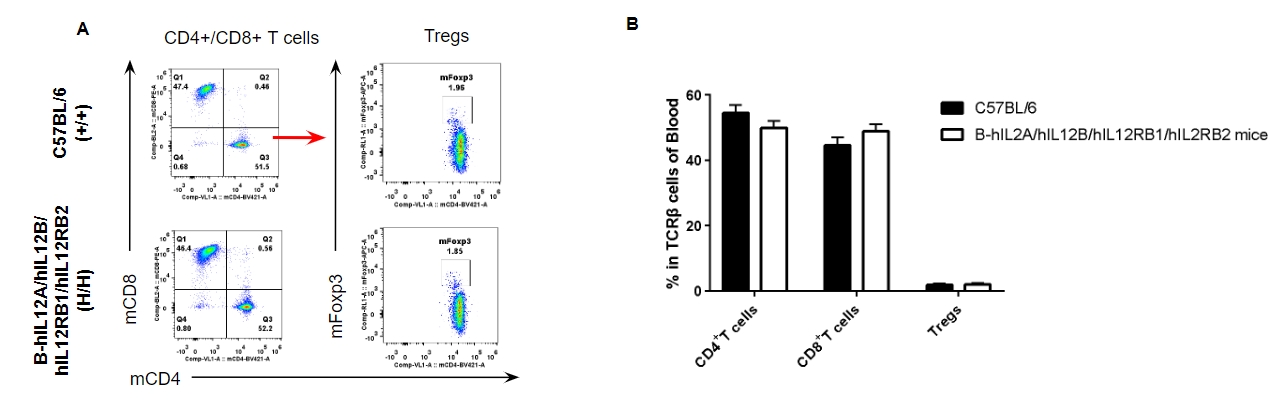
Analysis of blood T cell subpopulations by FACS. Blood were isolated from female C57BL/6 and B-hIL12A/hIL12B/hIL12RB1/hIL12RB2 mice (n=3, 6-week-old). Flow cytometry analysis of the blood was performed to assess leukocyte subpopulations. A. Representative FACS plots. Single live CD45+ cells were gated for TCRβ+ T cell population and used for further analysis as indicated here. B. Results of FACS analysis. The percent of CD4+ T cells, CD8+ T cells and Tregs in homozygous B-hIL12A/hIL12B/hIL12RB1/hIL12RB2 mice were similar to those in the C57BL/6 mice, demonstrating that the humanization does not change the overall development, differentiation or distribution of these cell types in blood. Values are expressed as mean ± SEM.
-
Blood routine test in B-hIL12A/hIL12B/hIL12RB1/hIL12RB2 mice

-
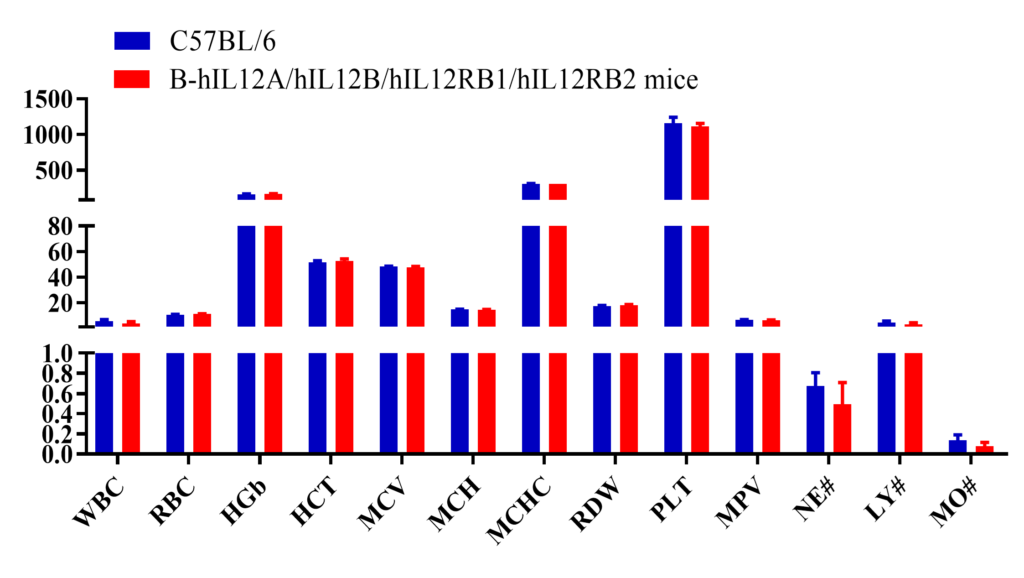
Complete blood count (CBC). Blood from female C57BL/6 and B-hIL12A/hIL12B/hIL12RB1/hIL12RB2 mice (n=6, 7 week-old) was collected and analyzed for CBC. The measurements of B-hIL12A/hIL12B/hIL12RB1/hIL12RB2 mice were similar to that in C57BL/6 mice, indicating that humanization does not change blood cell composition and morphology. Values are expressed as mean ± SEM.
-
Blood chemistry of B-hIL12A/hIL12B/hIL12RB1/hIL12RB2 mice

-
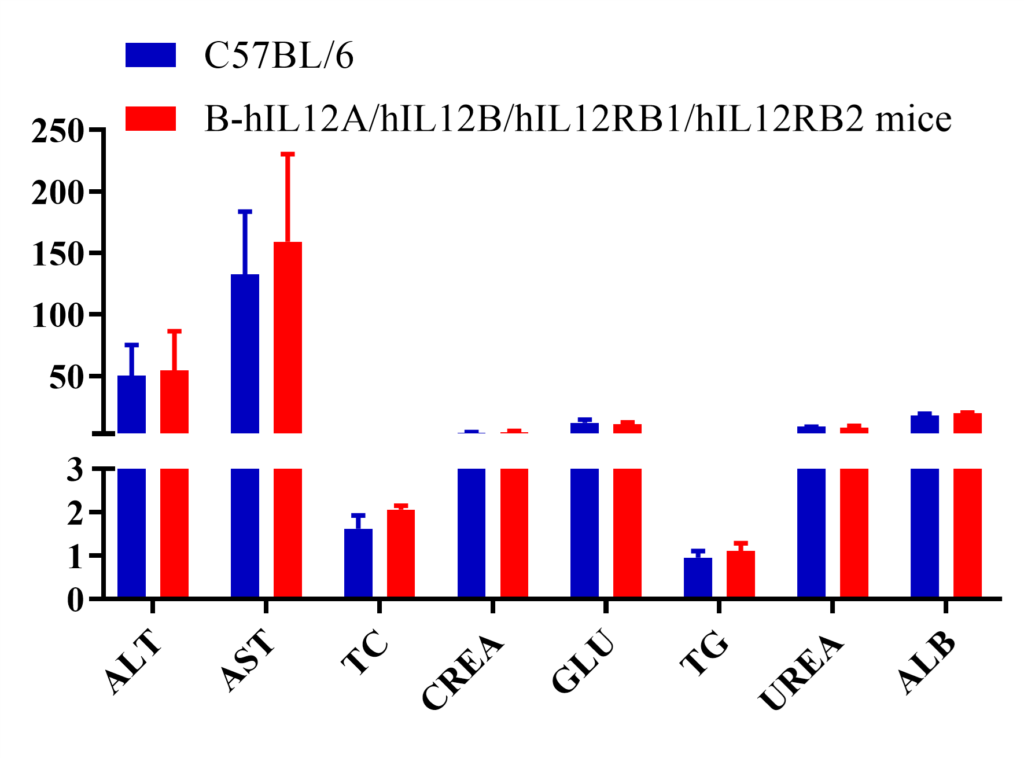
Blood chemistry tests in wild-type and humanized B-hIL12A/hIL12B/hIL12RB1/hIL12RB2 mice. Serum from the C57BL/6 and B-hIL12A/hIL12B/hIL12RB1/hIL12RB2 mice (n=6, 7 week-old) was collected and analyzed for levels of indicators. The measurements of B-hIL12A/hIL12B/hIL12RB1/hIL12RB2 mice were similar to that in C57BL/6 mice, indicating that humanization does not change the health of related tissues, such as liver. Values are expressed as mean ± SEM.
-
IL12 is a powerful partner in tumor immunotherapy

-
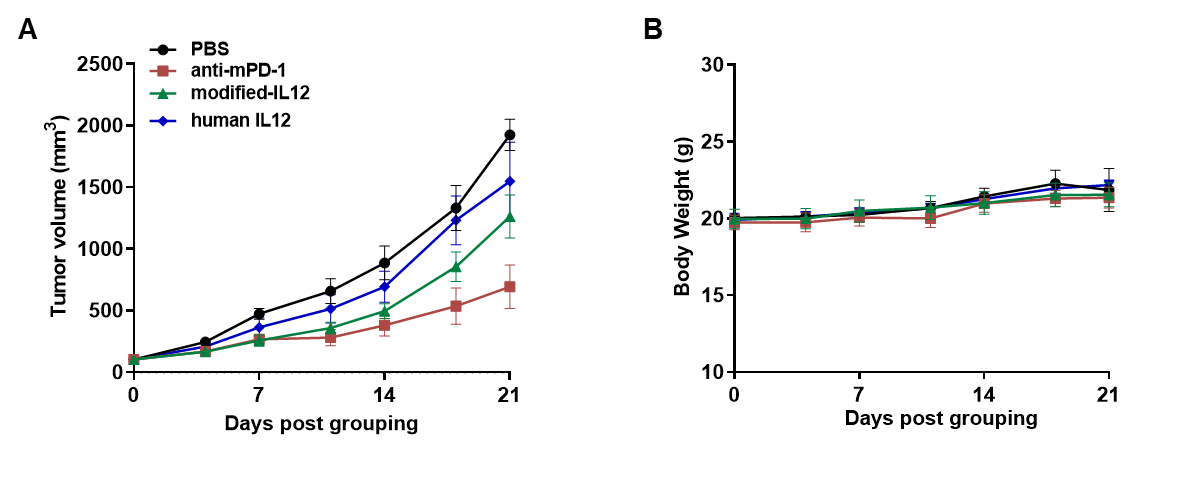
Antitumor activity of a modified human IL12 as an immune modulator in B-hIL12A/hIL12B/hIL12RB1/hIL12RB2 mice bearing colon cancer MC38 cells model. (A) Modified human IL12 from the cooperation customer inhibited MC38 tumor growth. Murine colon cancer MC38 cells were subcutaneously implanted into homozygous B-hIL12A/hIL12B/hIL12RB1/hIL12RB2 mice (female, 7-9 week-old, n=5). Mice were grouped when tumor volume reached approximately 100 mm3, at which time they were treated with drugs in the panel. (B) Body weight changes during treatment. As shown in panel A, the modified human IL12 shows inhibitory effects but the tumor recovers growth when the drug was stopped. Values are expressed as mean ± SEM.
Note: all drugs used in the experiment were provided by cooperative partner.
-
hIL12 induced IFN-γ production in activated T cell

-
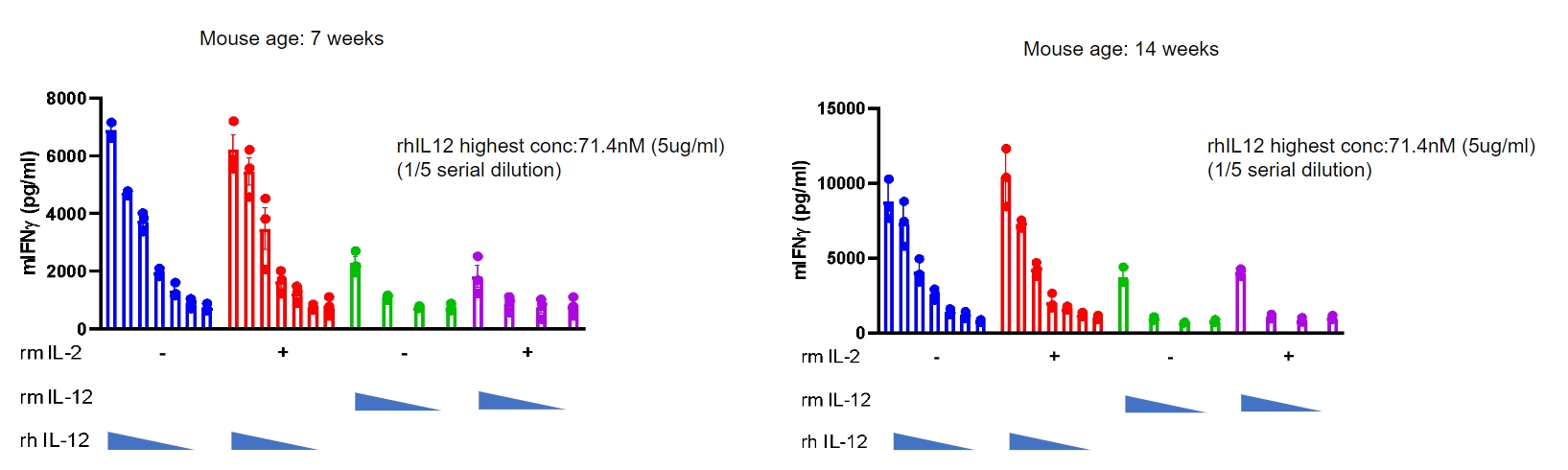
IL12 induced the IFN-γ production in CD3+ T cells sorted from splenocytes.
T cells were sorted from the splenocytes in homozygous B-hIL12A/hIL12B/hIL12RB1/hIL12RB2 mice (n=3), detected the IFN-γ production after incubation with bead-associated CD3 and CD28 mAbs and rmIL2, as well as rhIL12 with concentrations in the panel for 72h. Mouse IFN-γ induced by rhIL12 in a dose dependent. Homozygous humanized mice responded well to hIL-12 in vitro. (Co-validation data from a partner).
-
In vivo hIL-12 efficacy and toxicity test (MC38 tumor model)

-
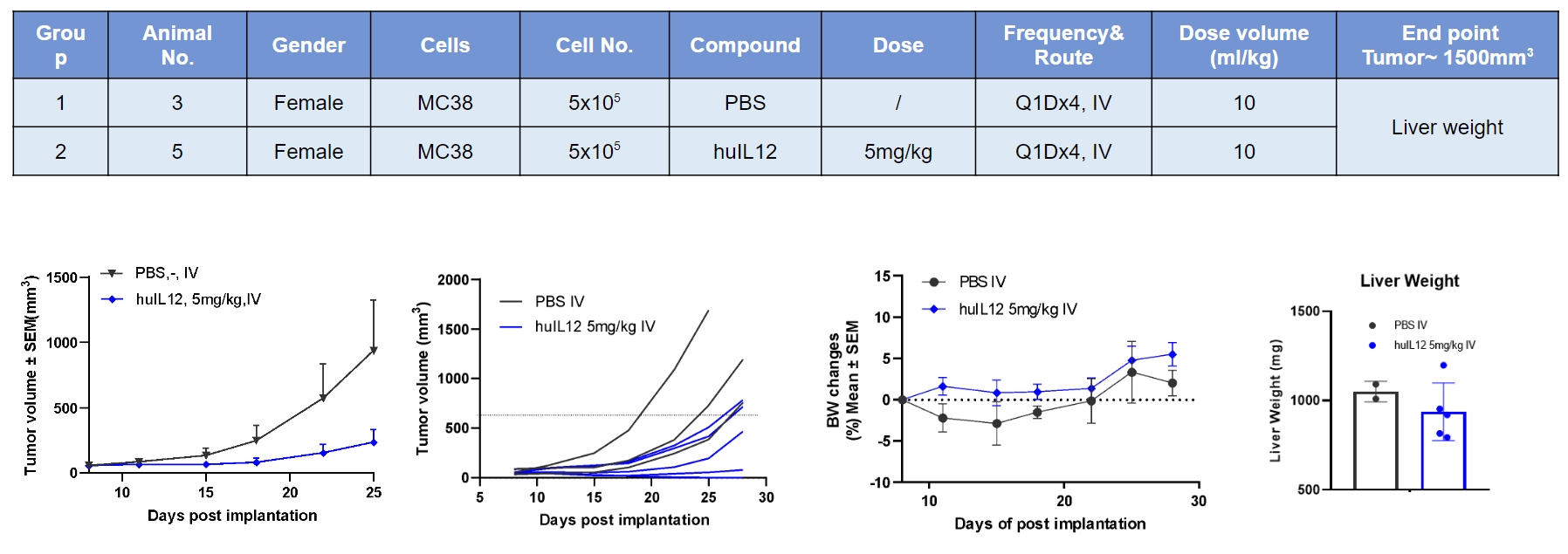
Human IL12 inhibited MC38 tumor growth. However, no body weight loss during treatment.
-
Summary

-
- mRNA expression in B-hIL12A/hIL12B/hIL12RB1/hIL12RB2 mice:
Human IL12A was detectable only in splenocytes of homozygous B-hIL12A/hIL12B/hIL12RB1/hIL12RB2 mice.
Human IL12B, IL12RB1 and IL12RB2 were only detectable in thymocytes of homozygous B-hIL12A/hIL12B/hIL12RB1/hIL12RB2 mice.
- Protein expression in B-hIL12A/hIL12B/hIL12RB1/hIL12RB2 mice:
Human IL12 (p70) was exclusively detectable in serum of homozygous B-hIL12A/hIL12B/hIL12RB1/hIL12RB2 mice.
- Leukocytes cell subpopulation analysis:
Humanized IL12A/IL12B/IL12RB1/IL12RB2 does not change the overall development, differentiation or distribution of immune cell types in spleen, lymph node and blood.
- Function analysis:
Mouse IFN-γ were increased after response to mIL-12 in wild-type mice and hIL-12 in homozygous B-hIL12A/hIL12B/hIL12RB1/hIL12RB2 mice (H/H) separately.
- In vivo efficacy:
Human IL12 inhibited MC38 tumor growth but no body weight loss during treatment.
-
Poster

-
AACR 2022: B-hIL12A/hIL12B/hIL12RB1/hIL12RB2: A Novel Animal Model for Generation of IL12 Therapies


