Basic Information
-
Gene targeting strategy

-
Gene targeting strategy for B-hIL1RACP mice. The chimeric IL1RAP chimeric coding sequence (human signal peptide, human extracellular domain, mouse transmembrane domain, and intracellular domain) was inserted after the 5’UTR of mouse Il1rap gene in B-hIL1RACP mice. The insertion disrupts the endogenous murine Il1rap gene, resulting in the absence of mouse transcripts.
-
mRNA expression analysis

-
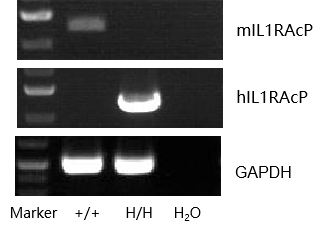
Strain specific analysis of IL1RAcP gene expression in wild type and B-hIL1RAcP mice by RT-PCR. Mouse IL1RAcP mRNA was detectable in liver of wild type mice (+/+). Human IL1RAcP mRNA was detectable only in B-hIL1RAcP mice (H/H) but not in wild type mice.
-
Protein expression analysis

-

Strain specific IL1RAcP expression analysis in homozygous B-hIL1RAcP mice by flow cytometry. Macrophages and monocytes were collected from wild type and homozygous B-hIL1RAcP mice (H/H) and analyzed by flow cytometry with species-specific anti-IL1RAcP antibody. Human IL1RAcP were exclusively detectable in homozygous B-hIL1RAcP but not wild type mice.
-
Functional verification

-




Splenocytes were collected from wild mice and homozygous B-hIL1RAcP mice and stimulated with IL1/IL33/IL36 protein. The expression of cytokines such as IL6, TNFa, KC/GRO, IL4, IFNg, IL5, and IL12p70 in splenocyte culture medium was detected after 48 hours. The results showed that murine IL1/IL33/IL36 protein effectively activated splenocytes in wild mice and triggered the secretion of related cytokines. Murine IL1B and murine IL33 proteins could effectively activate splenocytes in B-hIL1RAcP mice and trigger the secretion of related cytokines, while murine IL36 protein could not effectively activate B-hIL1RAcP mice splenocytes.


