Basic Information
C57BL/6-Il2tm1(IL2)BcgenIl2ratm1(IL2RA)Bcgenll2rbtm2(IL2RB)Bcgenll2rgtm2(IL2RG)Bcgen/Bcgen
-
Targeting strategy

-
Gene targeting strategy for B-hIL2/hIL2RA/hIL2RB/hIL2RG mice.
The mouse Il2 gene that encodes the full coding sequence was replaced by human IL2 full coding sequence.
The mouse Il2ra gene that encodes the extracellular domain were replaced by human IL2RA counterpart gene sequences.
The mouse Il2rb gene that encodes the extracellular domain were replaced by human IL2RB counterpart gene sequences.
The mouse Il2rg gene that encodes the full coding region sequences were replaced by human IL2RG counterpart gene sequences.
-
Protein expression analysis

-
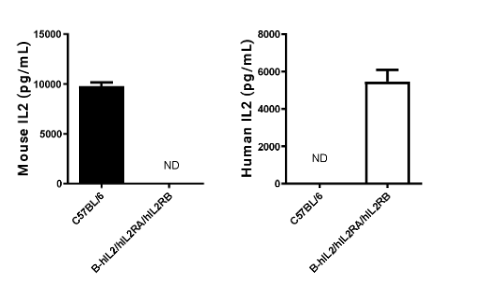
Strain specific IL2 expression analysis in homozygous B-hIL2/hIL2RA/hIL2RB/hIL2RG mice by ELISA. Serum was collected from wild-type mice and homozygous B-hIL2/hIL2RA/hIL2RB mice stimulated with anti-mCD3 and anti-mCD28 in vivo, and analyzed by ELISA with species-specific IL2 ELISA kit. Mouse IL2 was detectable in wild-type mice. Human IL2 was exclusively detectable in homozygous B-hIL2/hIL2RA/hIL2RB mice but not in wild-type mice.(ND: Not detectable)
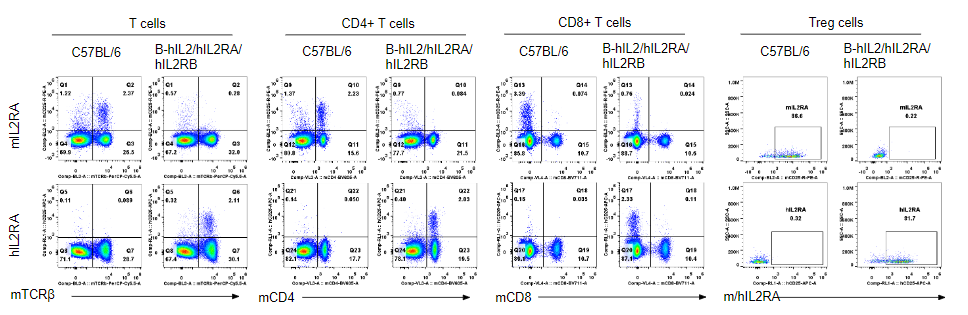
Strain specific IL2RA expression analysis in homozygous B-hIL2/hIL2RA/hIL2RB/hIL2RG mice by flow cytometry. Splenocytes were collected from wild-type mice and homozygous B-hIL2/hIL2RA/hIL2RB mice, and analyzed by flow cytometry with the species-specific anti-IL2RA antibody. Human IL2RA was exclusively detectable in T cells, CD4+ T cells and Treg cells of homozygous B-hIL2/hIL2RA/hIL2RB mice but not in wild-type mice. Mouse IL2RA was only detectable in these cells of wild-type mice. However, IL2RA was not detectable in CD8+ T cells in both these two mice.
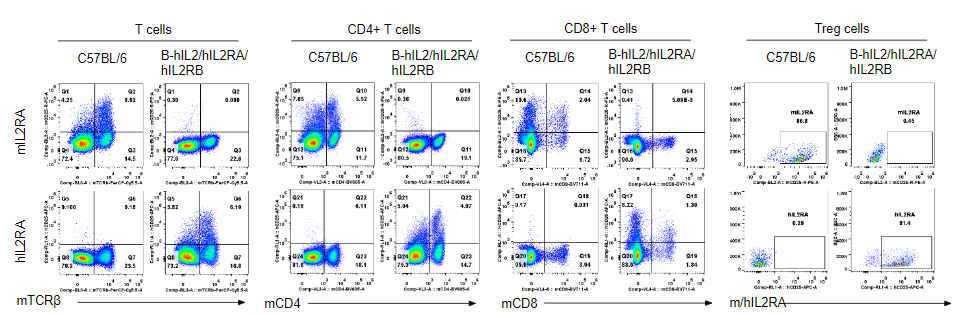
Strain specific IL2RA expression analysis in homozygous B-hIL2/hIL2RA/hIL2RB/hIL2RG mice by flow cytometry. Splenocytes were collected from wild-type mice and homozygous B-hIL2/hIL2RA/hIL2RB mice stimulated with anti-CD3ε in vivo, and analyzed by flow cytometry with the species-specific anti-IL2RA antibody. Human IL2RA was exclusively detectable in T cells, CD4+ T cells, CD8+ T cells and Treg cells of homozygous B-hIL2/hIL2RA/hIL2RB mice but not in wild-type mice. Mouse IL2RA was only detectable in these cells of wild-type mice.
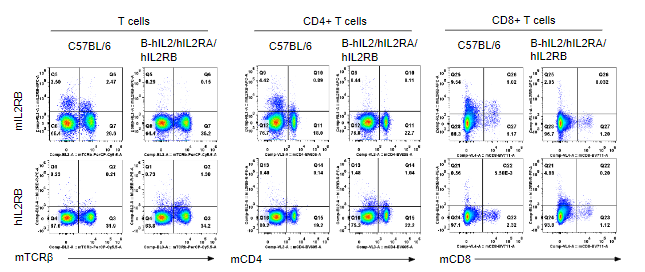
Strain specific IL2RB expression analysis in homozygous B-hIL2/hIL2RA/hIL2RB/hIL2RG mice by flow cytometry. Splenocytes were collected from wild-type mice and homozygous B-hIL2/hIL2RA/hIL2RB mice, and analyzed by flow cytometry with the species-specific anti-IL2RB antibody. Human IL2RB was exclusively detectable in T cells, CD4+ T cells and CD8+ T cells of homozygous B-hIL2/hIL2RA/hIL2RB mice but not in wild-type mice. Mouse IL2RA was only detectable in these cells of wild-type mice.
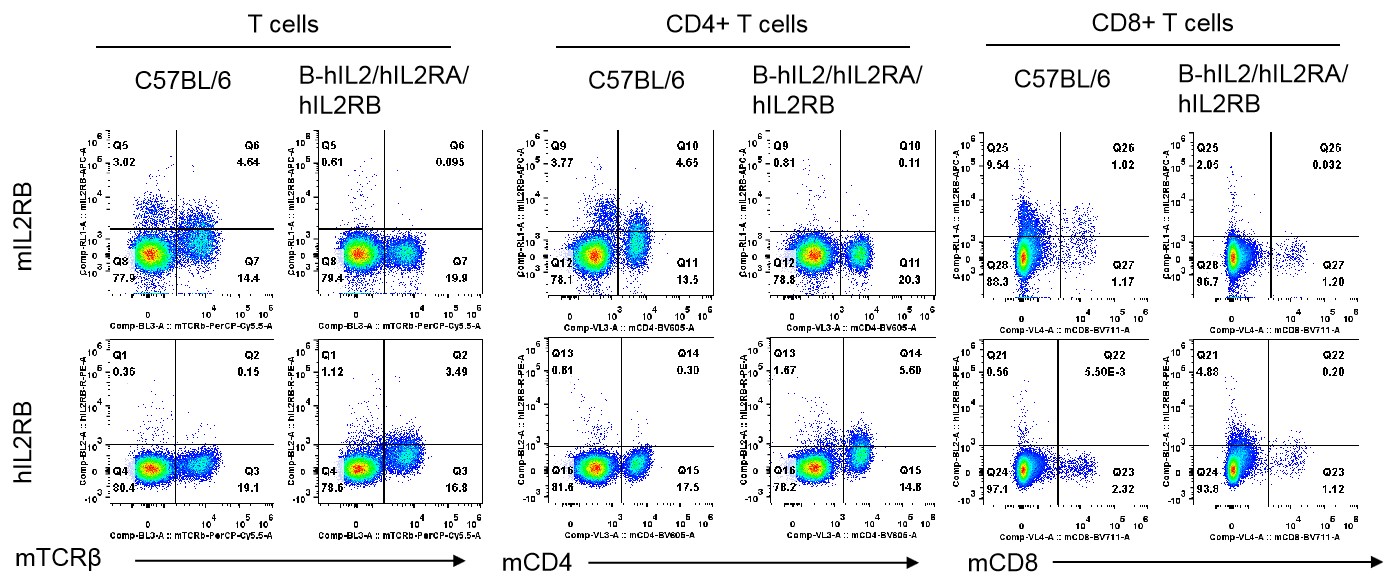
Strain specific IL2RB expression analysis in homozygous B-hIL2/hIL2RA/hIL2RB/hIL2RG mice by flow cytometry. Splenocytes were collected from wild-type mice and homozygous B-hIL2/hIL2RA/hIL2RB mice stimulated with anti-CD3ε in vivo, and analyzed by flow cytometry with the species-specific anti-IL2RB antibody. Human IL2RB was exclusively detectable in T cells, CD4+ T cells and CD8+ T cells of homozygous B-hIL2/hIL2RA/hIL2RB mice but not in wild-type mice. Mouse IL2RA was only detectable in these cells of wild-type mice.
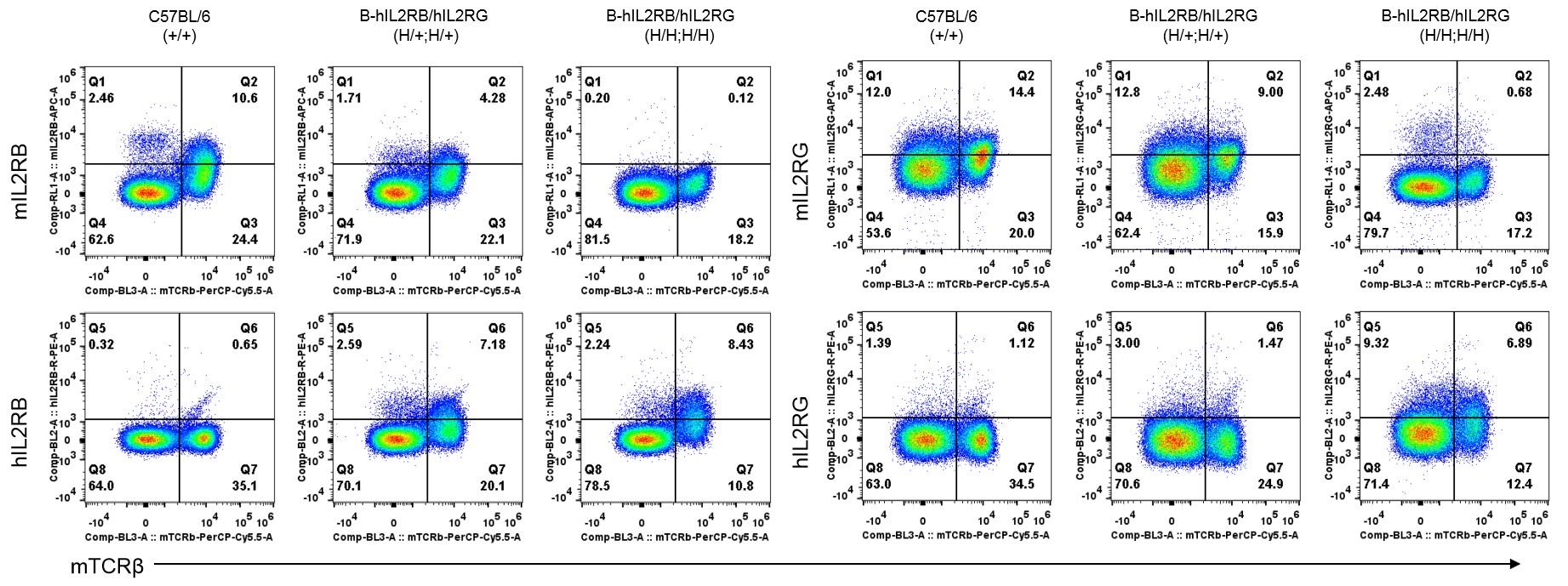
Strain specific IL2RB and IL2RG expression analysis in heterozygous and homozygous B-hIL2/hIL2RA/hIL2RB/hIL2RG mice by flow cytometry. Splenocytes were collected from wild-type mice and B-hIL2RB/hIL2RG mice, and analyzed by flow cytometry with species-specific antibody. Human IL2RB was detectable in heterozygous (H/+;H/+) and homozygous B-hIL2RB/hIL2RG mice (H/H;H/H). Human IL2RG was too low to be detectable in heterozygous, but detectable in homozygous B-hIL2RB/hIL2RG mice.
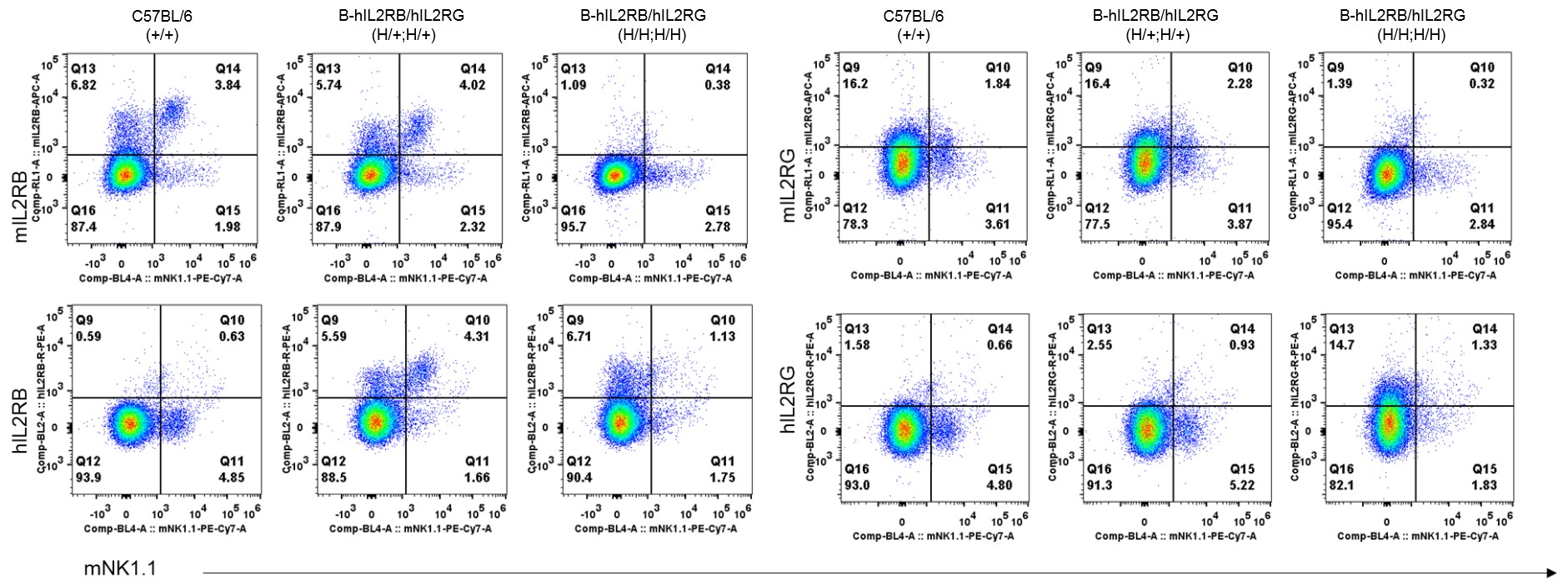
Strain specific IL2RB and IL2RG expression analysis in heterozygous and homozygous B-hIL2/hIL2RA/hIL2RB/hIL2RG mice by flow cytometry. Splenocytes were collected from wild-type mice and B-hIL2RB/hIL2RG mice, and analyzed by flow cytometry with species-specific antibody. Human IL2RB was detectable in heterozygous (H/+;H/+) and homozygous (H/H;H/H) B-hIL2RB/hIL2RG mice. Human IL2RG was too low to be detectable in heterozygous mice, and it was unable to judge the IL2RG expression in NK cells, because NK cells were diminished in homozygous B-hIL2RB/hIL2RG mice.
-
Phosphorylation of STAT5 induced by IL2

-
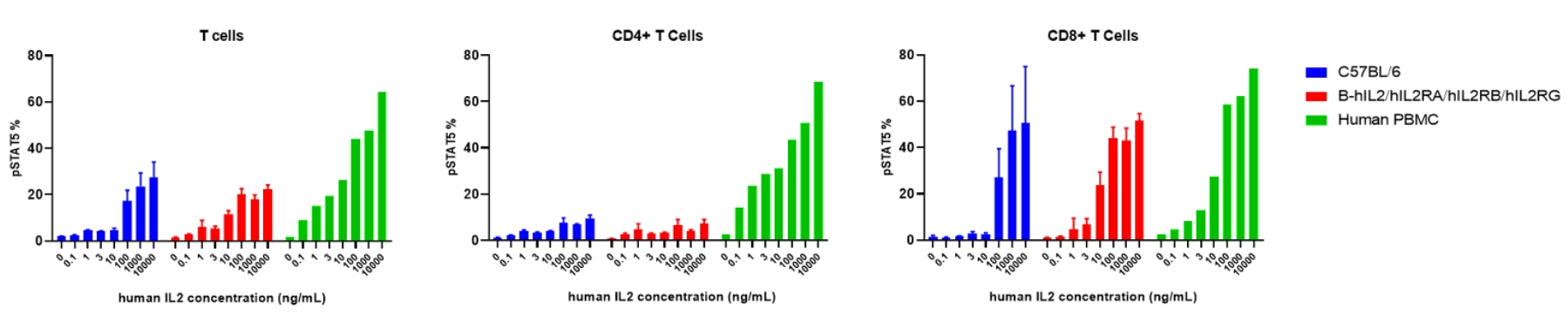
Intracellular phosphorylation of pSTAT5 analysis in splenocytes by flow cytometry. Splenocytes were harvested and analyzed for pSTAT5 induction in the T cell subtypes of wild type C57BL/6 mice and B-hIL2/hIL2RA/hIL2RB/hIL2RG mice. IL2 induced pSTAT5 in T cells with a dose dependent manner. Specifically induced high level of pSTAT5 in CD8+T cells. At 10ng/mL concentration, human IL2 induced higher pSTAT5 in T cells of B-hIL2/hIL2RA/hIL2RB/hIL2RG mice than that in wild type C57BL/6 mice.
-
Frequency of leukocyte subpopulations in spleen

-
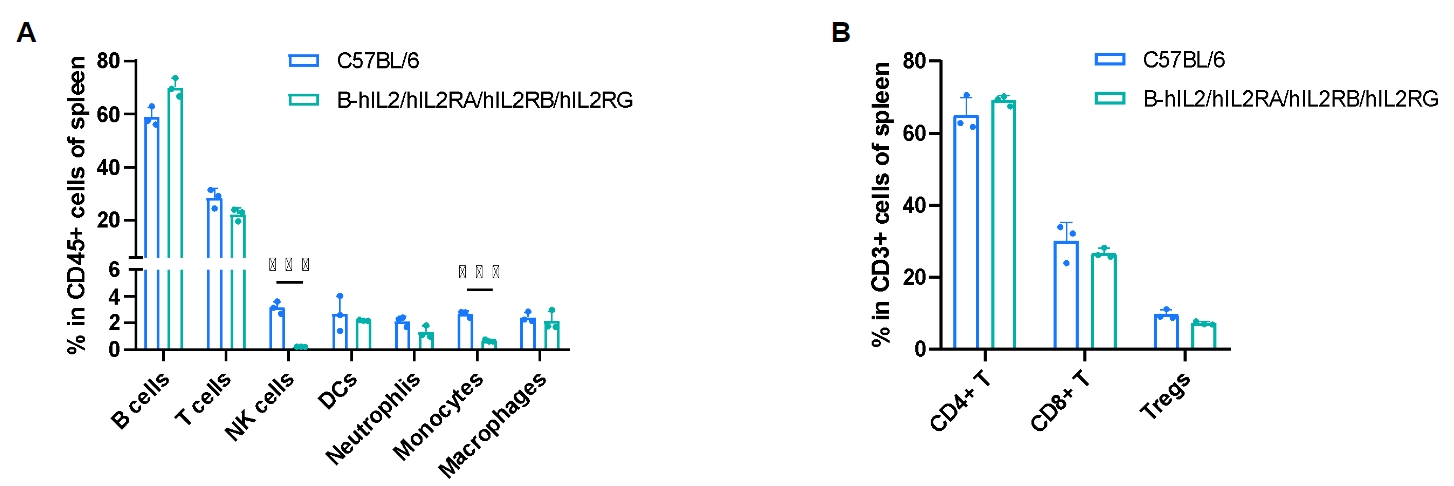
Frequency of leukocyte subpopulations in spleen by flow cytometry. Splenocytes were isolated from male wild-type C57BL/6 mice (n=3, 8-week-old) and homozygous B-hIL2/hIL2RA/IL2RB/IL2RG mice (n=3, 9-week-old). A. Flow cytometry analysis of the splenocytes was performed to assess the frequency of leukocyte subpopulations. B. Frequency of T cell subpopulations. Percentages of T cells, B cells, dendritic cells, neutrophils, macrophages, CD4+ T cells, CD8+ T cells and Tregs in B-hIL2/hIL2RA/IL2RB/IL2RG mice were similar to those in C57BL/6 mice. NK cells and monocytes decreased in humanized mice. Values are expressed as mean ± SD. Significance was determined by two-way ANOVA test. *P < 0.05, **P < 0.01, ***p < 0.001.
-
Frequency of leukocyte subpopulations in blood

-
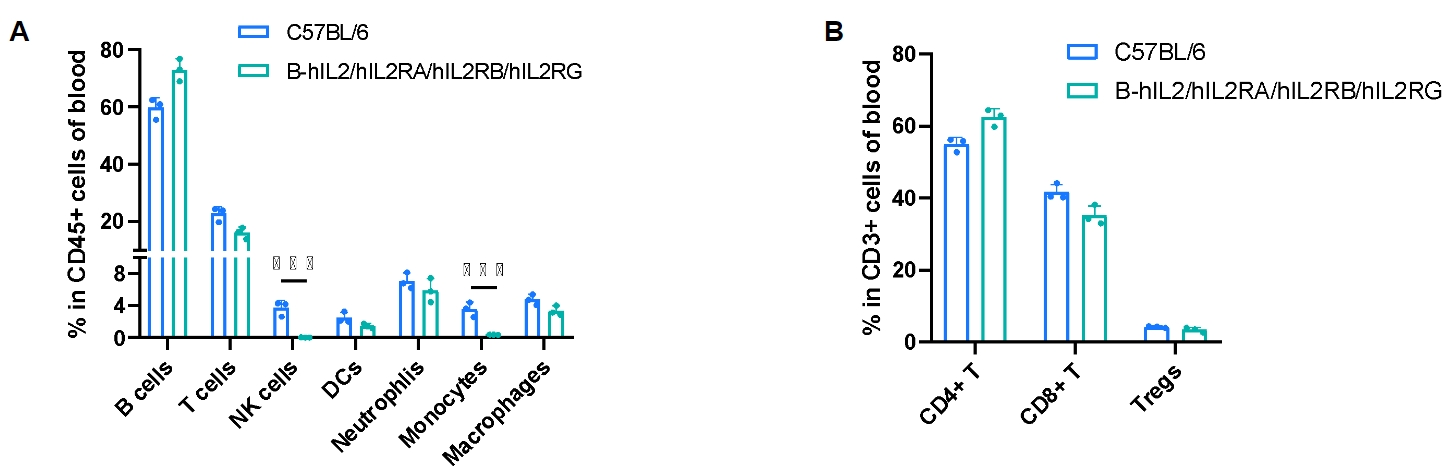
Frequency of leukocyte subpopulations in blood by flow cytometry. Blood cells were isolated from male wild-type C57BL/6 mice (n=3, 8-week-old) and homozygous B-hIL2/hIL2RA/IL2RB/IL2RG mice (n=3, 9-week-old). A. Flow cytometry analysis of the blood cells was performed to assess the frequency of leukocyte subpopulations. B. Frequency of T cell subpopulations. Percentages of T cells, B cells, dendritic cells, neutrophils, macrophages, CD4+ T cells, CD8+ T cells and Tregs in B-hIL2/hIL2RA/IL2RB/IL2RG mice were similar to those in C57BL/6 mice. NK cells and monocytes decreased in humanized mice. Values are expressed as mean ± SD. Significance was determined by two-way ANOVA test. *P < 0.05, **P < 0.01, ***p < 0.001.
-
Frequency of leukocyte subpopulations in lymph nodes

-
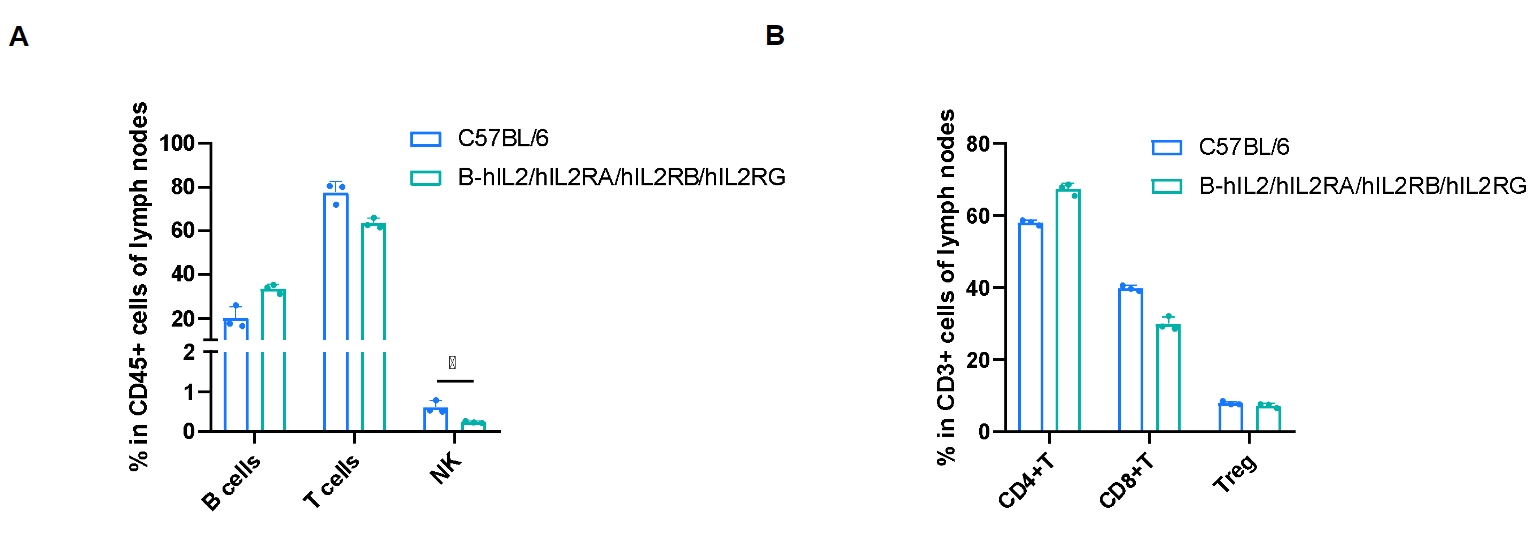
Frequency of leukocyte subpopulations in lymph nodes by flow cytometry. Lymph nodes cells were isolated from male wild-type C57BL/6 mice (n=3, 8-week-old) and homozygous B-hIL2/hIL2RA/IL2RB/IL2RG mice (n=3, 9-week-old). A. Flow cytometry analysis of the lymph nodes cells was performed to assess the frequency of leukocyte subpopulations. B. Frequency of T cell subpopulations. Percentages of T cells, B cells, CD4+ T cells, CD8+ T cells and Tregs in B-hIL2/hIL2RA/IL2RB/IL2RG mice were similar to those in C57BL/6 mice. NK cells decreased in humanized mice. Values are expressed as mean ± SD. Significance was determined by two-way ANOVA test. *P < 0.05, **P < 0.01, ***p < 0.001.
-
Frequency of leukocyte subpopulations in thymus

-
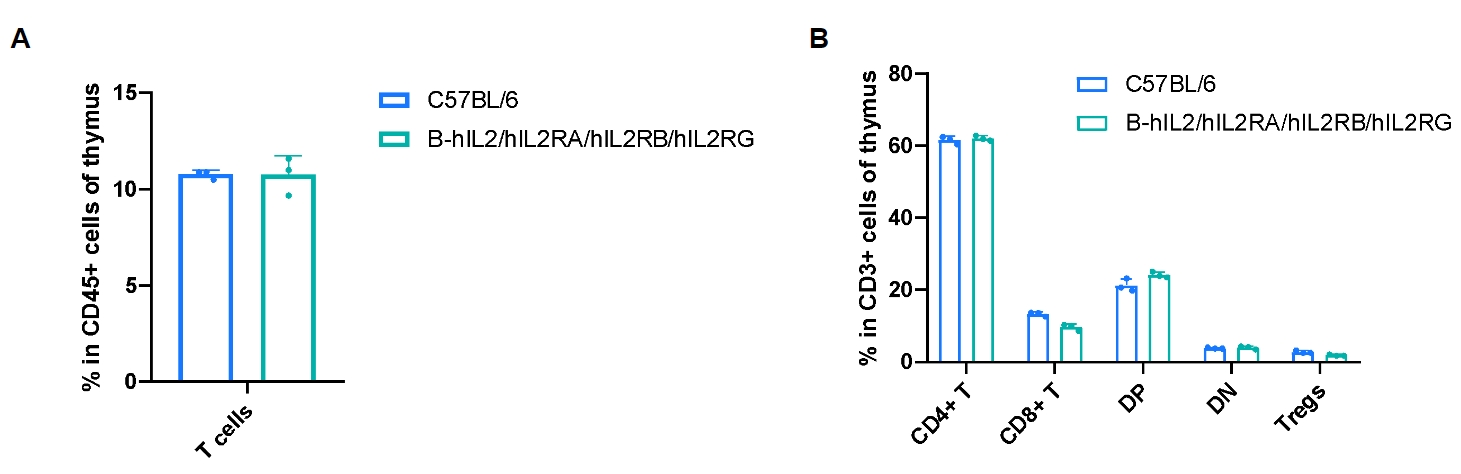
Frequency of leukocyte subpopulations in thymus by flow cytometry. Thymus cells were isolated from male wild-type C57BL/6 mice (n=3, 8-week-old) and homozygous B-hIL2/hIL2RA/IL2RB/IL2RG mice (n=3, 9-week-old). A. Flow cytometry analysis of the thymus cells was performed to assess the frequency of leukocyte subpopulations. B. Frequency of T cell subpopulations. Percentages of T cells, CD4+ T cells, CD8+ T cells and Tregs in B-hIL2/hIL2RA/IL2RB/IL2RG mice were similar to those in C57BL/6 mice. Values are expressed as mean ± SD. Significance was determined by two-way ANOVA test. *P < 0.05, **P < 0.01, ***p < 0.001.
-
Summary

-
Protein expression analysis:
Human IL2, IL2RA and IL2RB were detectable in B-hIL2/hIL2RA/hIL2RB mice.
Human IL2RB and IL2RG were detectable in B-hIL2RB/hIL2RG mice.
Phosphorylation of STAT5 induced by IL2:
Human IL2 induce pSTAT5 in B-hIL2/hIL2RA/hIL2RB/hIL2RG mice. The IL2RA, IL2RB and IL2RG humanization did not change the signaling pathway.
Leukocytes cell subpopulation analysis:
- Percentages of T cells, B cells, dendritic cells, neutrophils, and macrophages in humanized mice were similar to those in C57BL/6 mice.
- NK cells had a deficiency in spleen, blood and lymph node of humanized mice.
- Monocytes decrease in spleen, blood and lymph node of humanized mice.
- T cell subtypes in thymus, spleen, blood and lymph node of humanized mice were similar to that in C57BL/6 mice.


