Basic Information
-
Targeting strategy

-
Gene targeting strategy for B-hCCR9 mice. The mouse Ccr9 gene that encode the full length of coding region were replaced by human CCR9 coding sequence in B-hCCR9 mice.
-
Protein expression analysis

-
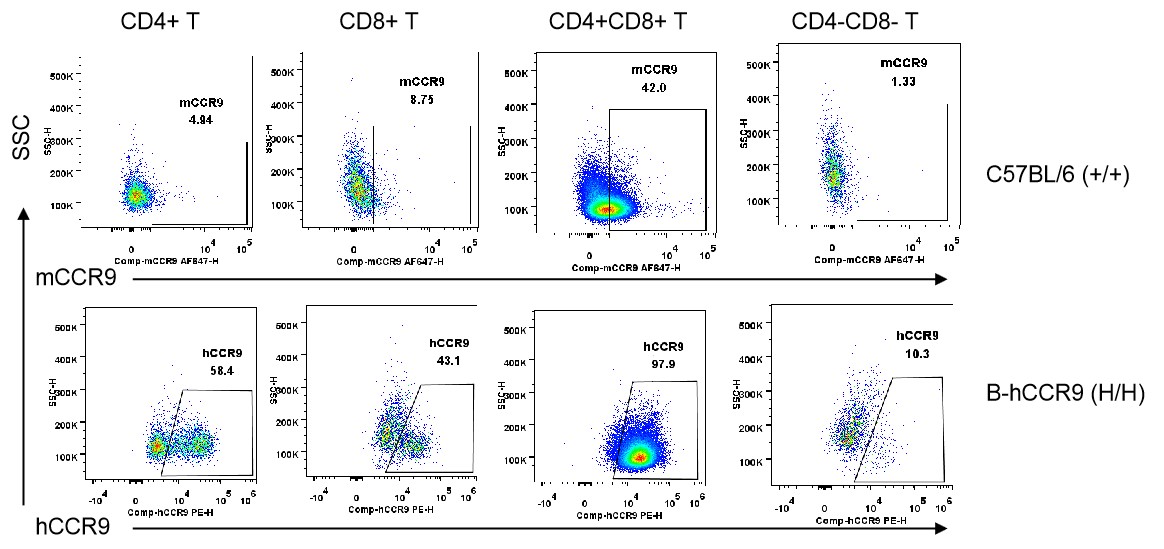
Strain specific CCR9 expression analysis in homozygous B-hCCR9 mice by flow cytometry. Thymocytes were collected from WT and homozygous B-hCCR9 (H/H) mice, and analyzed by flow cytometry with species-specific anti-CCR9 antibody. Mouse CCR9 was detectable in wild-type C57BL/6 mice (+/+). Human CCR9 was exclusively detectable in homozygous B-hCCR9 mice (H/H) but not in wild-type C57BL/6 mice.
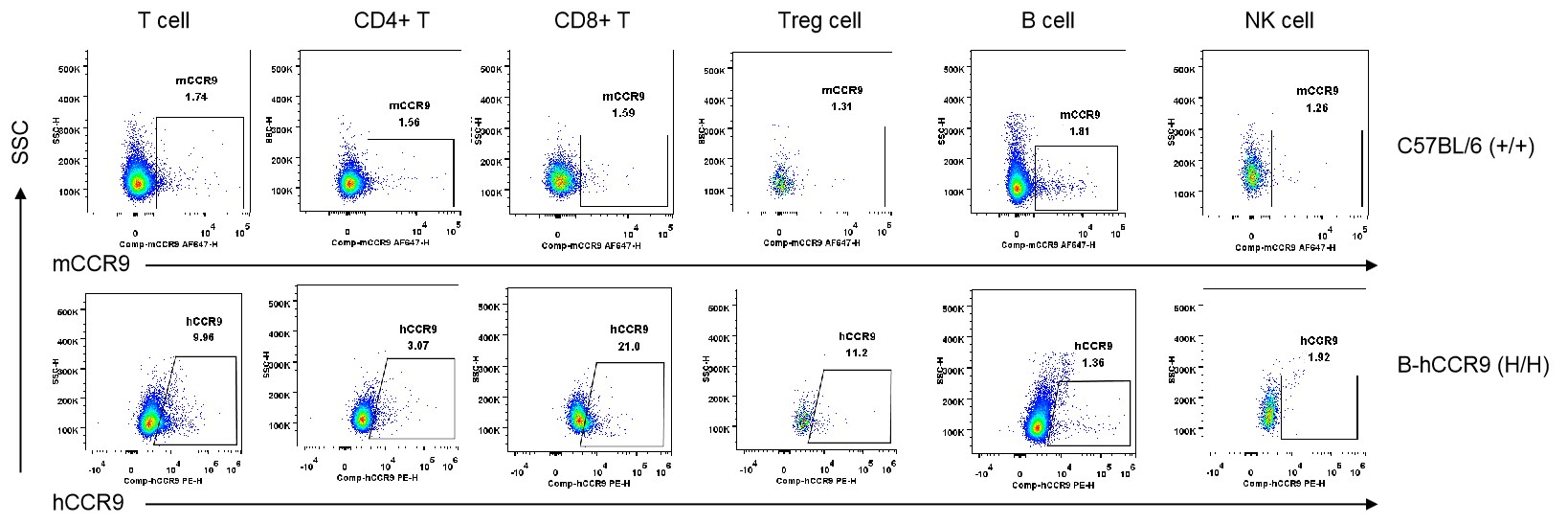
Strain specific CCR9 expression analysis in homozygous B-hCCR9 mice by flow cytometry. Splenocytes were collected from wild-type C57BL/6 mice (+/+) and homozygous B-hCCR9 mice (H/H), and analyzed by flow cytometry with species-specific anti-CCR9 antibody. Human CCR9 was exclusively detectable in T cells and CD8+ T cells of homozygous B-hCCR9 mice but not in CD4+ T cells, Tregs, B cells and NK cells.
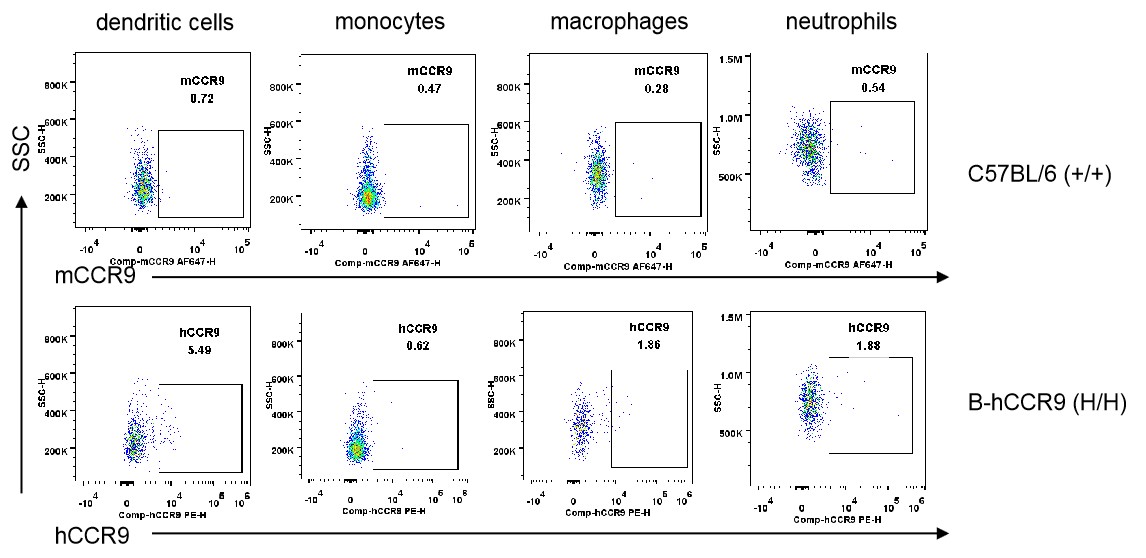
Strain specific CCR9 expression analysis in homozygous B-hCCR9 mice by flow cytometry. Splenocytes were collected from wild-type C57BL/6 mice (+/+) and homozygous B-hCCR9 mice (H/H), and analyzed by flow cytometry with species-specific anti-CCR9 antibody. Human CCR9 was not detectable in dendritic cells, monocytes, macrophages and neutrophils.
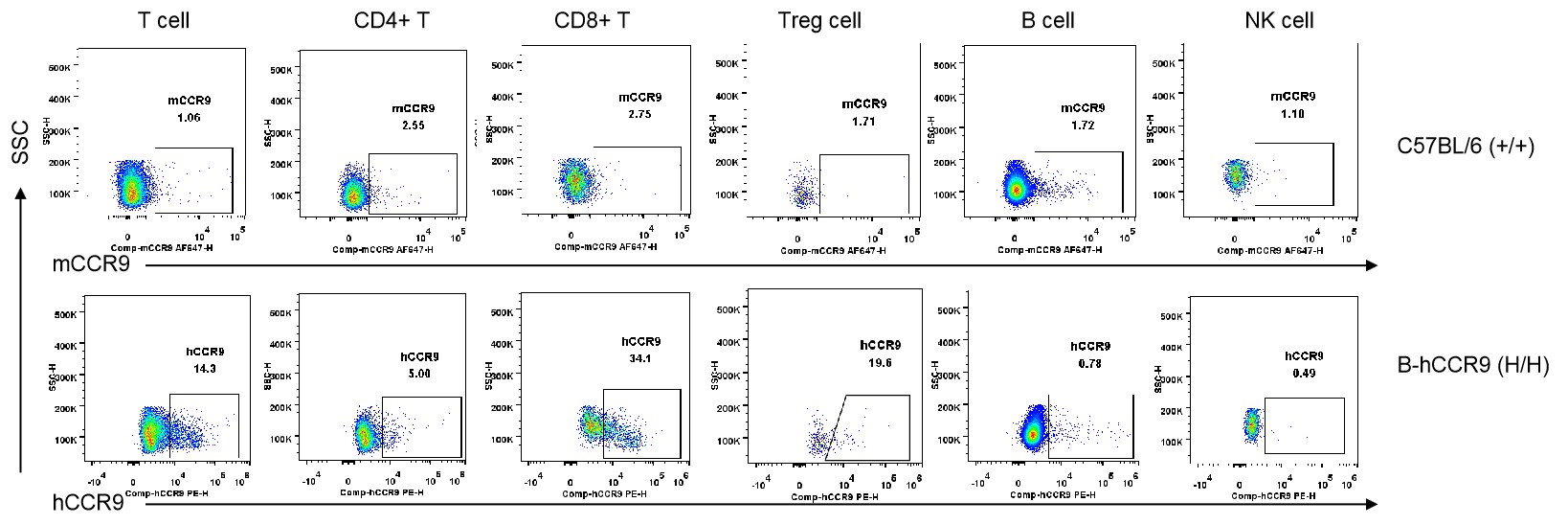
Strain specific CCR9 expression analysis in homozygous B-hCCR9 mice by flow cytometry. Blood cells were collected from wild-type C57BL/6 mice (+/+) and homozygous B-hCCR9 mice (H/H), and analyzed by flow cytometry with species-specific anti-CCR9 antibody. Human CCR9 was exclusively detectable in T cells and CD8+ T cells of homozygous B-hCCR9 mice but not in CD4+ T cells, Tregs, B cells and NK cells.
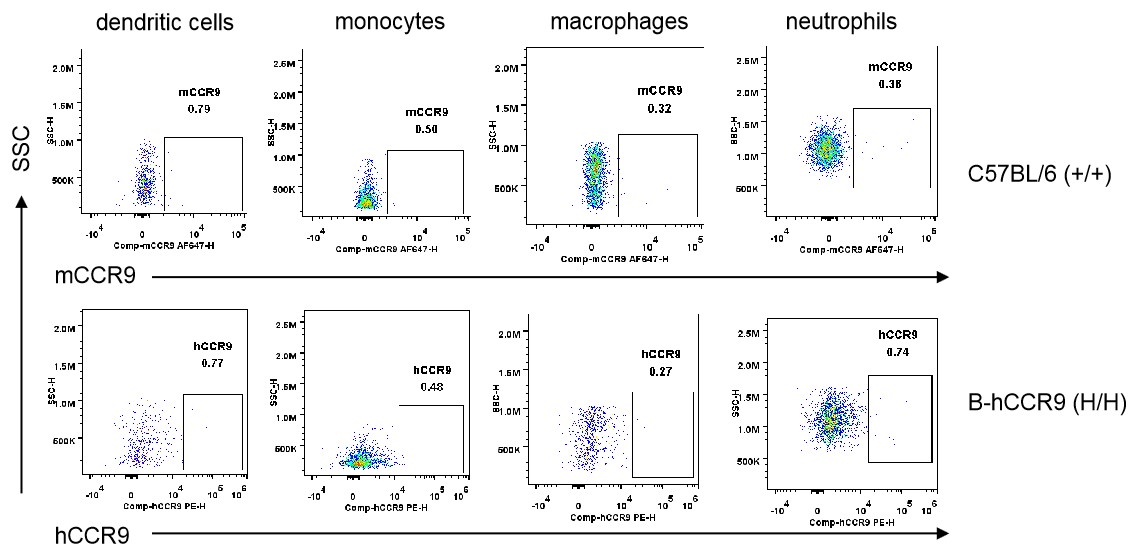
Strain specific CCR9 expression analysis in homozygous B-hCCR9 mice by flow cytometry. Blood cells were collected from wild-type C57BL/6 mice (+/+) and homozygous B-hCCR9 mice (H/H), and analyzed by flow cytometry with species-specific anti-CCR9 antibody. Human CCR9 was not detectable in dendritic cells, monocytes, macrophages and neutrophils.
-
mRNA expression analysis

-
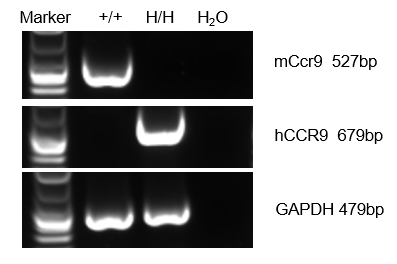
Strain specific analysis of CCR9 gene expression in wild-type mice and hCCR9 mice by RT-PCR. Mouse Ccr9 mRNA was detectable only in thymocytes of wild-type mice (+/+). Human CCR9 mRNA was detectable only in homozygous B-hCCR9 mice (H/H) but not in wild-type C57BL/6 mice.
-
Analysis of leukocytes cell subpopulation in thymus

-
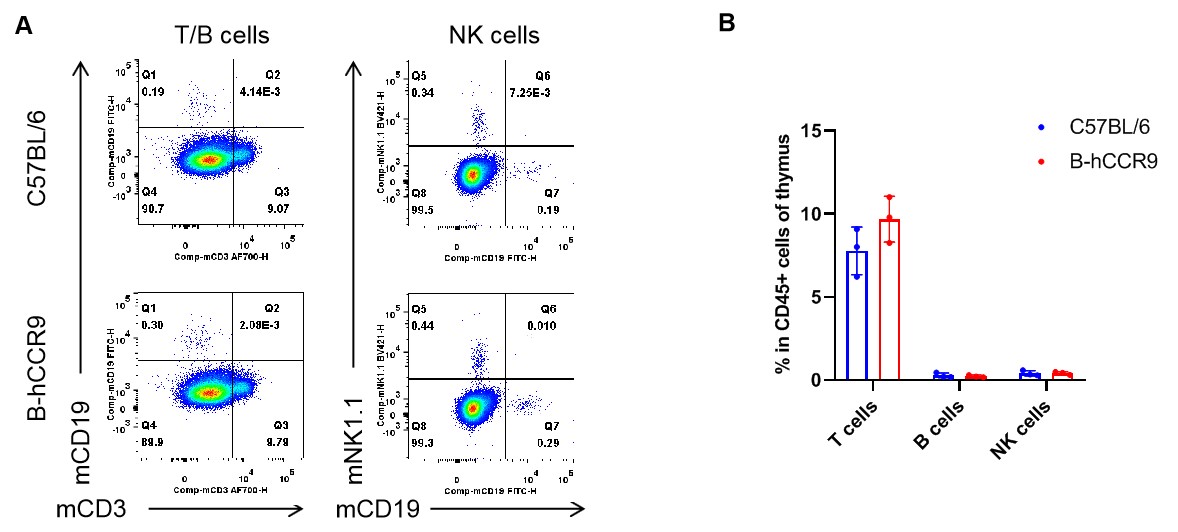
Analysis of thymus leukocyte subpopulations by flow cytometry. Thymus were isolated from female C57BL/6 and B-hCCR9 mice (n=3, 6-week-old). Flow cytometry analysis of the leukocytes was performed to assess leukocyte subpopulations. A. Representative FACS plots. Single live cells were gated for CD45+ population and used for further analysis as indicated here. B. Results of FACS analysis. The percent of T cells, B cells and NK cells in homozygous B-hCCR9 mice were similar to those in the C57BL/6 mice, demonstrating that introduction of hCCR9 in place of its mouse counterpart does not change the overall development, differentiation or distribution of these cell types in thymus. Values are expressed as mean ± SEM.
-
Analysis of T cell subpopulation in thymus

-
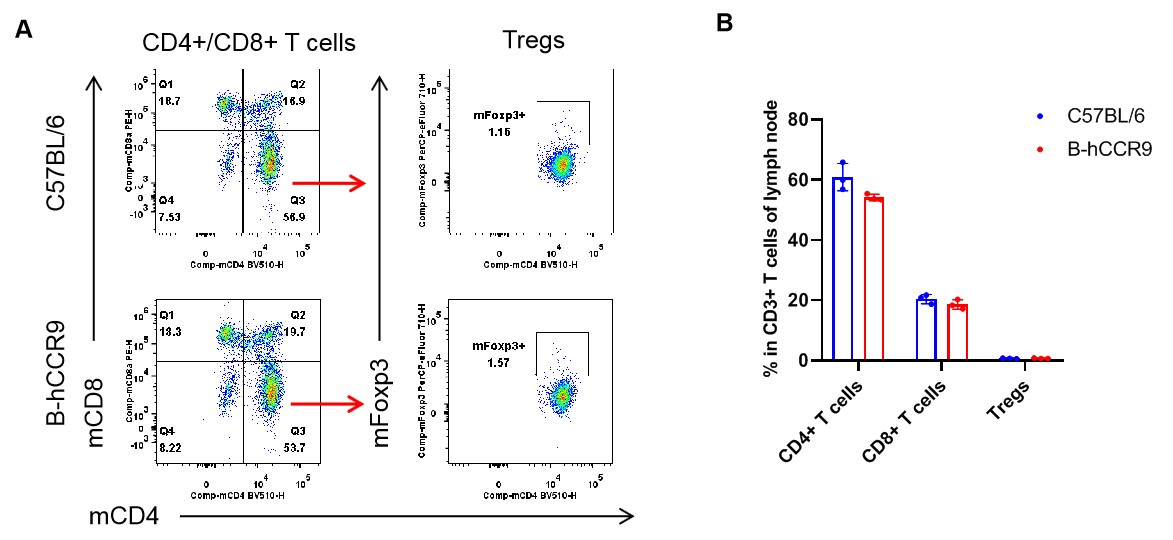
Analysis of thymus T cell subpopulations by flow cytometry. Thymus were isolated from female C57BL/6 and B-hCCR9 mice (n=3, 6-week-old). Flow cytometry analysis of the leukocytes was performed to assess leukocyte subpopulations. A. Representative FACS plots. Single live CD45+ cells were gated for CD3+ T cell population and used for further analysis as indicated here. B. Results of FACS analysis. The percent of CD8+ T cells, CD4+ T cells, and Tregs in homozygous B-hCCR9 mice were similar to those in the C57BL/6 mice, demonstrating that introduction of hCCR9 in place of its mouse counterpart does not change the overall development, differentiation or distribution of these T cell subtypes in thymus. Values are expressed as mean ± SEM.
-
Analysis of leukocytes cell subpopulation in spleen

-
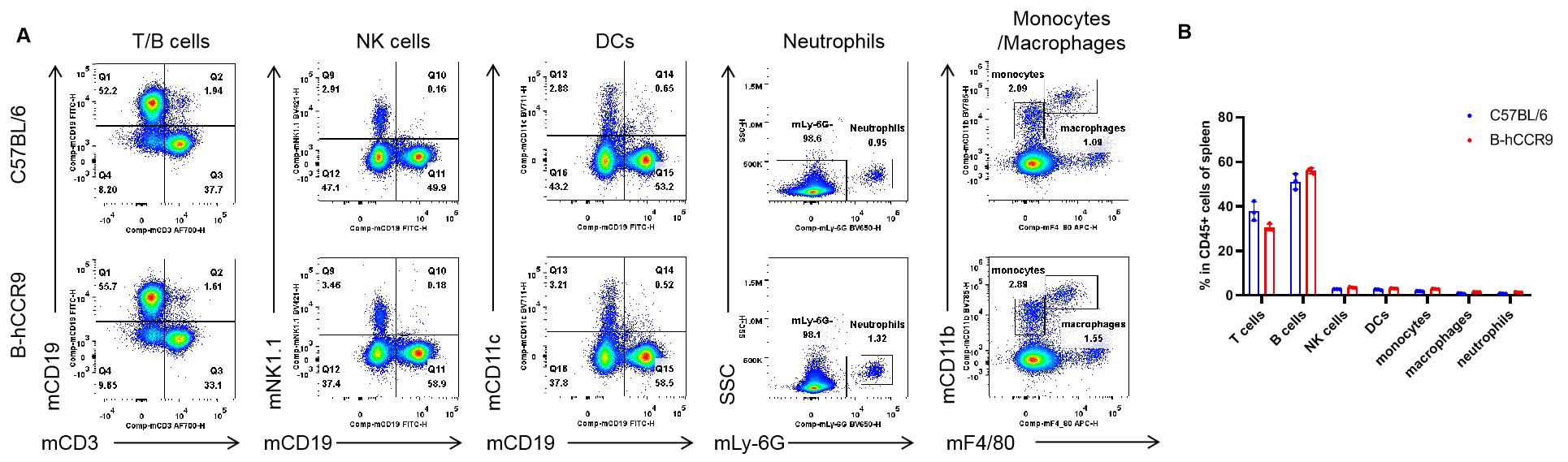
Analysis of spleen leukocyte subpopulations by flow cytometry. Splenocytes were isolated from female C57BL/6 and B-hCCR9 mice (n=3, 6-week-old). Flow cytometry analysis of the splenocytes was performed to assess leukocyte subpopulations. A. Representative FACS plots. Single live cells were gated for the CD45+ population and used for further analysis as indicated here. B. Results of FACS analysis. Percent of T cells, B cells, NK cells, DCs, neutrophils, monocytes and macrophages in homozygous B-hCCR9 mice were similar to those in the C57BL/6 mice, demonstrating that CCR9 humanized does not change the overall development, differentiation or distribution of these cell types in spleen. Values are expressed as mean ± SEM.
-
Analysis of T cell subpopulation in spleen

-
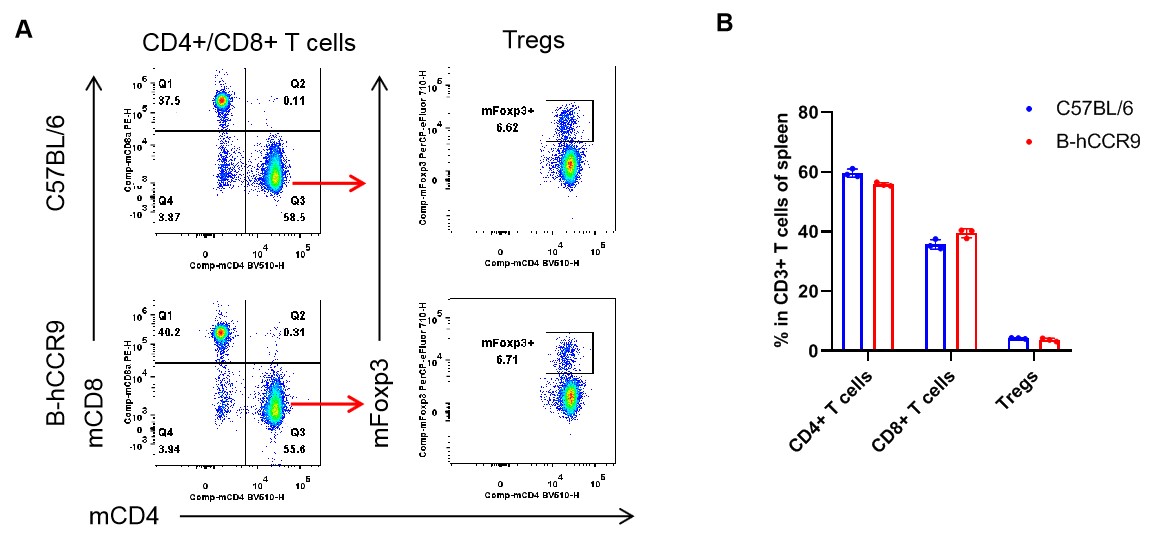
Analysis of spleen T cell subpopulations by flow cytometry. Splenocytes were isolated from female C57BL/6 and B-hCCR9 mice (n=3, 6-week-old). Flow cytometry analysis of the splenocytes was performed to assess leukocyte subpopulations. A. Representative FACS plots. Single live CD45+ cells were gated for CD3+ T cell population and used for further analysis as indicated here. B. Results of FACS analysis. The percent of CD8+ T cells, CD4+ T cells, and Tregs in homozygous B-hCCR9 mice were similar to those in the C57BL/6 mice, demonstrating that introduction of hCCR9 in place of its mouse counterpart does not change the overall development, differentiation or distribution of these T cell subtypes in spleen. Values are expressed as mean ± SEM.
-
Analysis of leukocytes cell subpopulation in blood

-
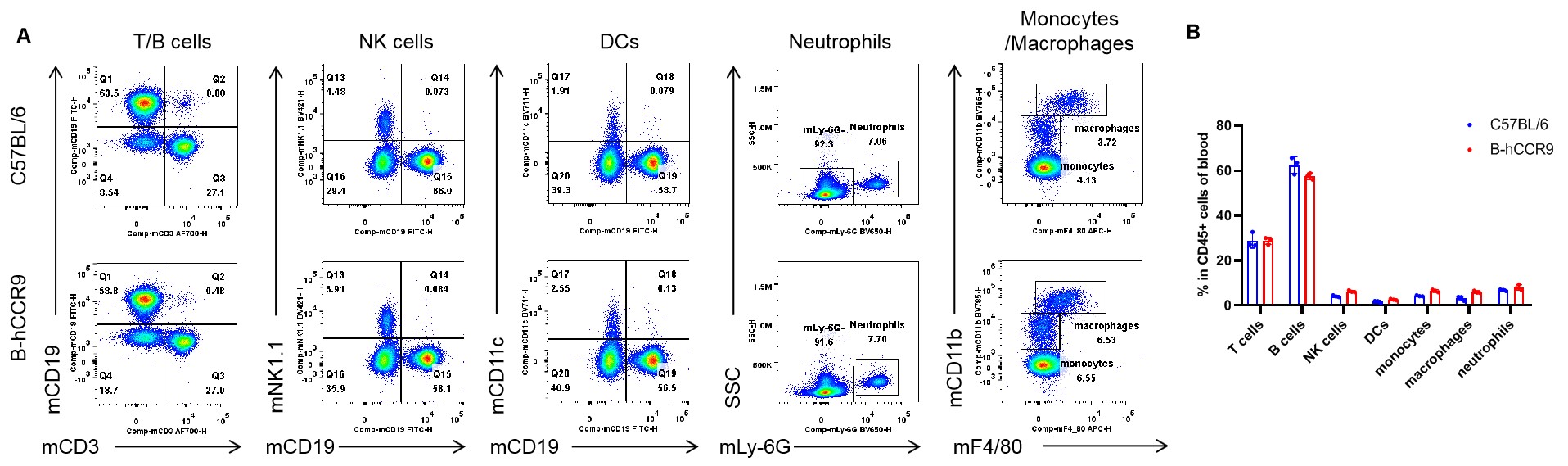
Analysis of blood leukocyte subpopulations by flow cytometry. Blood were isolated from female C57BL/6 and B-hCCR9 mice (n=3, 6-week-old). Flow cytometry analysis of the leukocytes was performed to assess leukocyte subpopulations. A. Representative FACS plots. Single live cells were gated for the CD45+ population and used for further analysis as indicated here. B. Results of FACS analysis. Percent of T cells, B cells, NK cells, DCs, neutrophils, monocytes and macrophages in homozygous B-hCCR9 mice were similar to those in the C57BL/6 mice, demonstrating that CCR9 humanized does not change the overall development, differentiation or distribution of these cell types in blood. Values are expressed as mean ± SEM.
-
Analysis of T cell subpopulation in blood

-
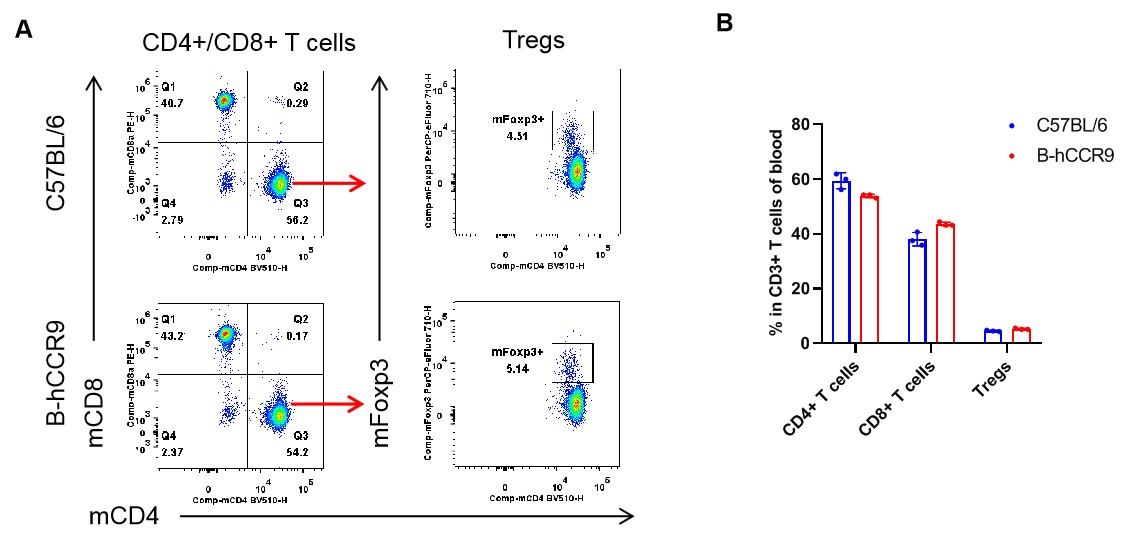
Analysis of blood T cell subpopulations by flow cytometry. Blood were isolated from female C57BL/6 and B-hCCR9 mice (n=3, 6-week-old). Flow cytometry analysis of the leukocytes was performed to assess leukocyte subpopulations. A. Representative FACS plots. Single live CD45+ cells were gated for CD3+ T cell population and used for further analysis as indicated here. B. Results of FACS analysis. The percent of CD8+ T cells, CD4+ T cells, and Tregs in homozygous B-hCCR9 mice were similar to those in the C57BL/6 mice, demonstrating that introduction of hCCR9 in place of its mouse counterpart does not change the overall development, differentiation or distribution of these T cell subtypes in blood. Values are expressed as mean ± SEM.
-
Summary

-
mRNA expression analysis:
Human CCR9 mRNA was detectable in B-hCCR9 mice (H/H) mice but not in wild-type mice.
Protein expression analysis:
Human CCR9 was exclusively detectable in homozygous B-hCCR9 mice (H/H) but not in wild-type C57BL/6 mice.


