Basic Information
-
Targeting strategy

-
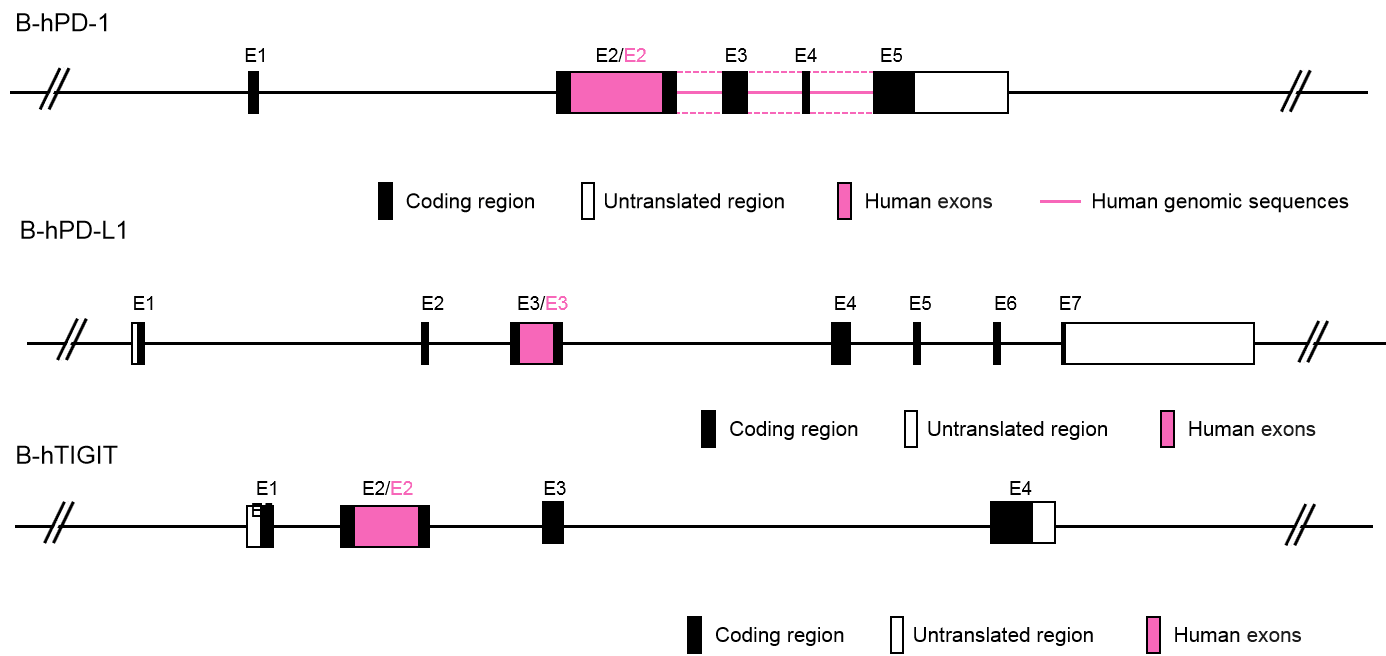
Gene targeting strategy for B-hPD-1/hPD-L1/hTIGIT mice. The exon 2 of mouse PD-1 gene that encodes the extracellular domain was replaced by human PD-1 exon 2 in B-hPD-1/hPD-L1/hTIGIT mice. The exon 3 of mouse pdl1 gene that encode the extracellular domain was replaced by human PD-L1 exon 3 in B-hPD-1/hPD-L1/hTIGIT mice. The exon 2 of mouse tigit gene that encodes the extracellular domain was replaced by human TIGIT exon 2 in B-hPD-1/hPD-L1/hTIGIT mice.
-
Protein expression analysis

-
Protein expression analysis

Strain specific PD-1, PD-L1 and TIGIT expression analysis in homozygous B-hPD-1/hPD-L1/hTIGIT mice by flow cytometry. Splenocytes were collected from WT and homozygous B-hPD-1/hPD-L1/hTIGIT (H/H) mice analyzed by flow cytometry with species-specific anti-PD-1 antibody. Mouse PD-1, PD-L1 and TIGIT were detectable in WT mice. Human PD-1, PD-L1 and TIGIT were exclusively detectable in homozygous B-hPD-1/hPD-L1/hTIGIT but not WT mice.

Strain specific PD-1, PD-L1 and TIGIT expression analysis in homozygous B-hPD-1/hPD-L1/hTIGIT mice by flow cytometry. Splenocytes were collected from WT and homozygous B-hPD-1/hPD-L1/hTIGIT (H/H) mice after stimulation with anti-CD3ε in vivo (7.5 μg/mice), and without stimulation, and analyzed by flow cytometry with species-specific anti-PD-1 antibody. Mouse PD-1, PD-L1 and TIGIT were detectable in WT mice. Human PD-1, PD-L1 and TIGIT were exclusively detectable in homozygous B-hPD-1/hPD-L1/hTIGIT but not WT mice. After anti-CD3ε stimulation, hPD-1 protein expression was significantly increased.
-
Immune cell profile

-
Analysis of Blood leukocytes cell subpopulations—no anti-mCD3

Analysis of Spleen leukocytes cell subpopulations—no anti-mCD3
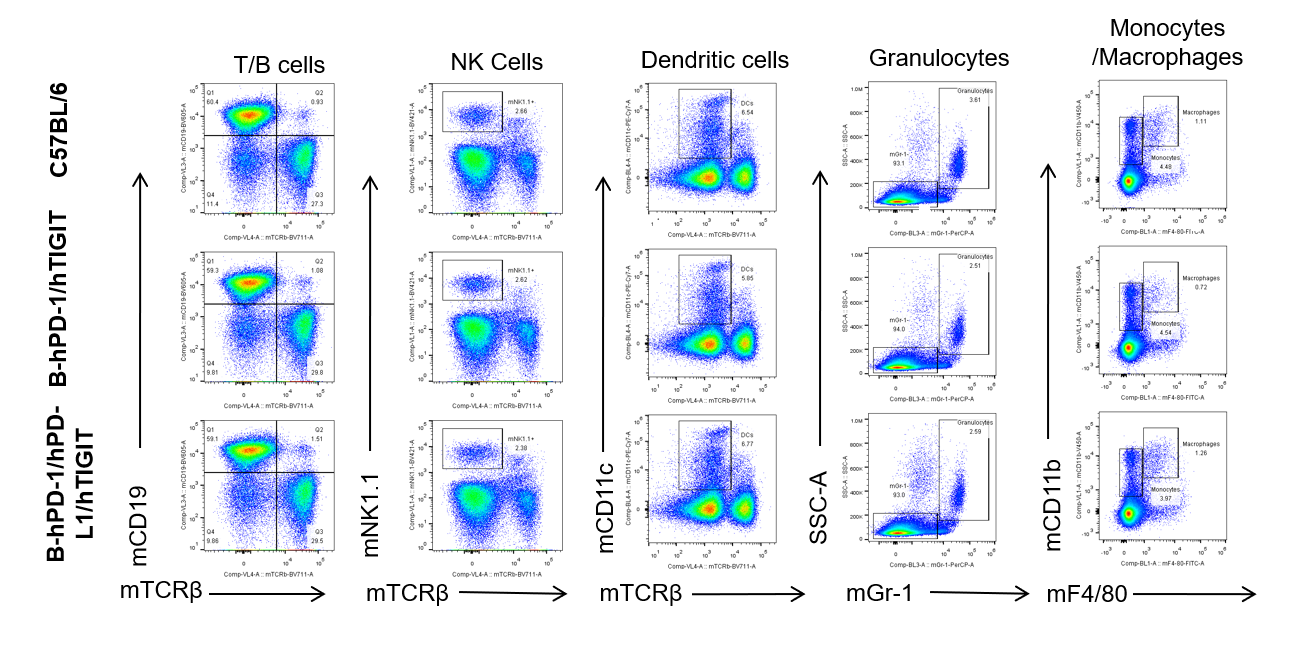
Analysis of lymph node leukocytes cell subpopulations—no anti-mCD3
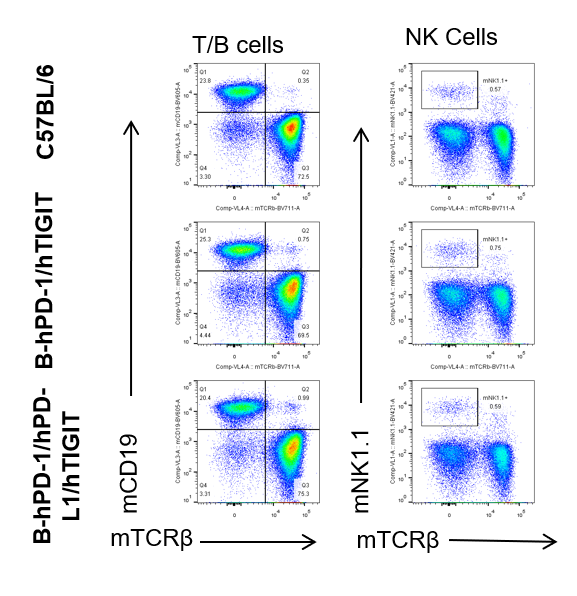
Analysis of Spleen leukocytes cell subpopulation—anti-mCD3

Analysis of lymph node leukocytes cell subpopulations—anti-mCD3
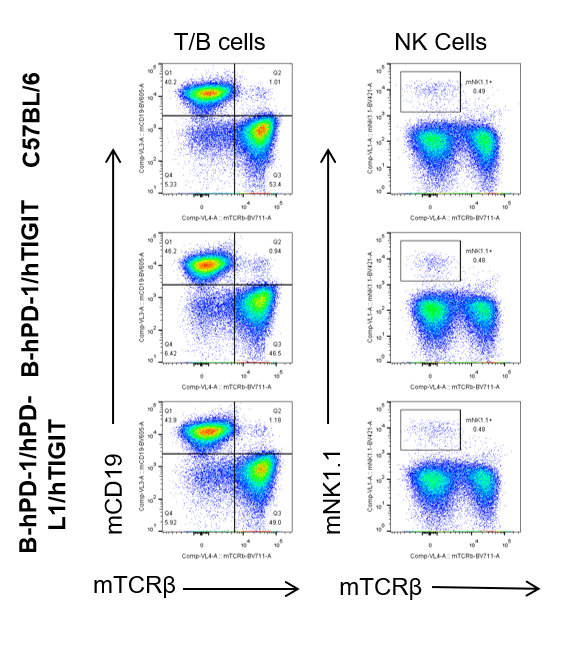
Analysis of blood, Spleen and lymph node leukocytes cell subpopulation
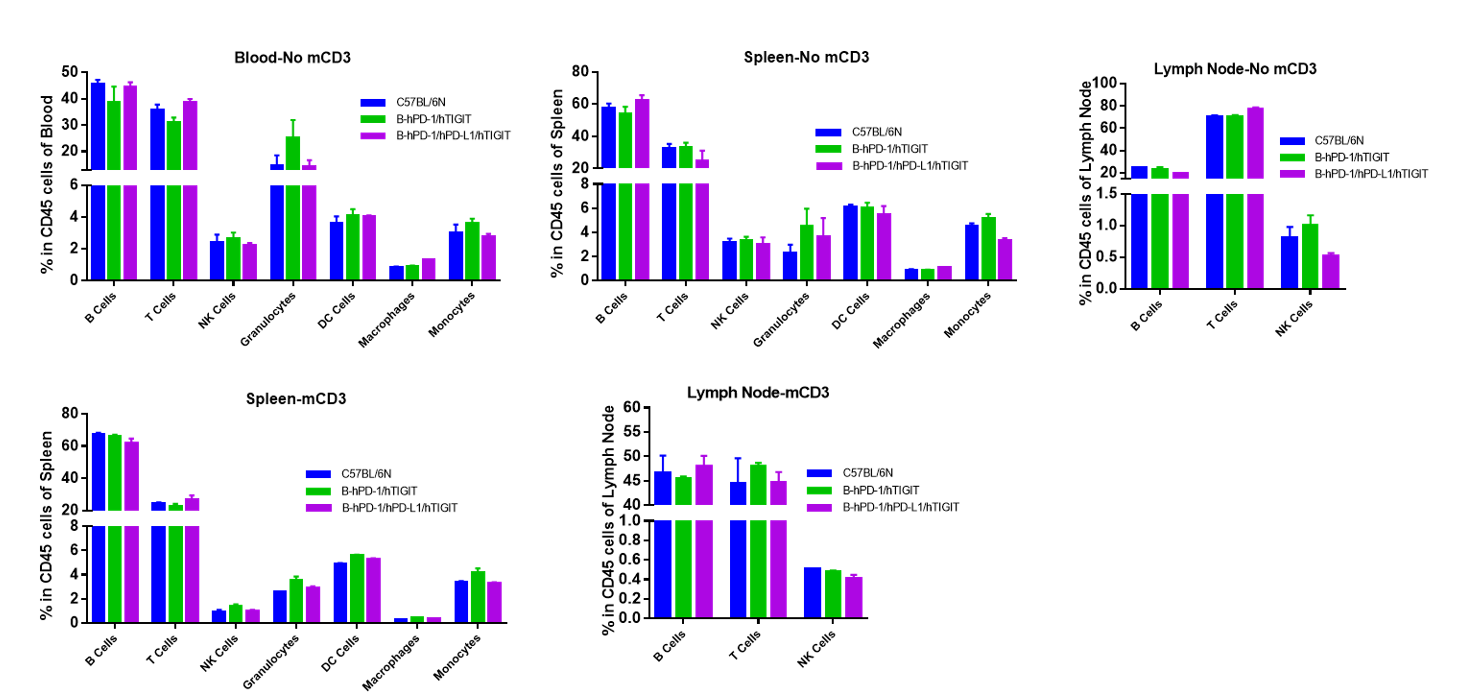
Analysis of blood, spleen and lymph node leukocytes cell subpopulations by FACS
Blood, spleen and lymph node leukocytes cell were isolated from female mice in the panel(n=3, 6 week-old). Flow cytometry analysis was performed to assess leukocyte subpopulations. Percent of T, B, NK, Granulocytes, Monocyte, DC and macrophage cells in homozygous B-hPD-1/hPD-L1/hTIGIT mice were similar to those in the C57BL/6 mice and B-hPD-1/hTIGIT mice both at rest and in stimulated with anti-CD3ε in vivo, demonstrating that the humanized mouse does not change the overall development, differentiation or distribution of these cell types in blood, spleen and lymph node.
Analysis of blood, Spleen and lymph node T cells subpopulations—no anti-mCD3
Analysis of Spleen and lymph node T cells subpopulations—anti-mCD3
Analysis of blood, Spleen and lymph node T cells subpopulations

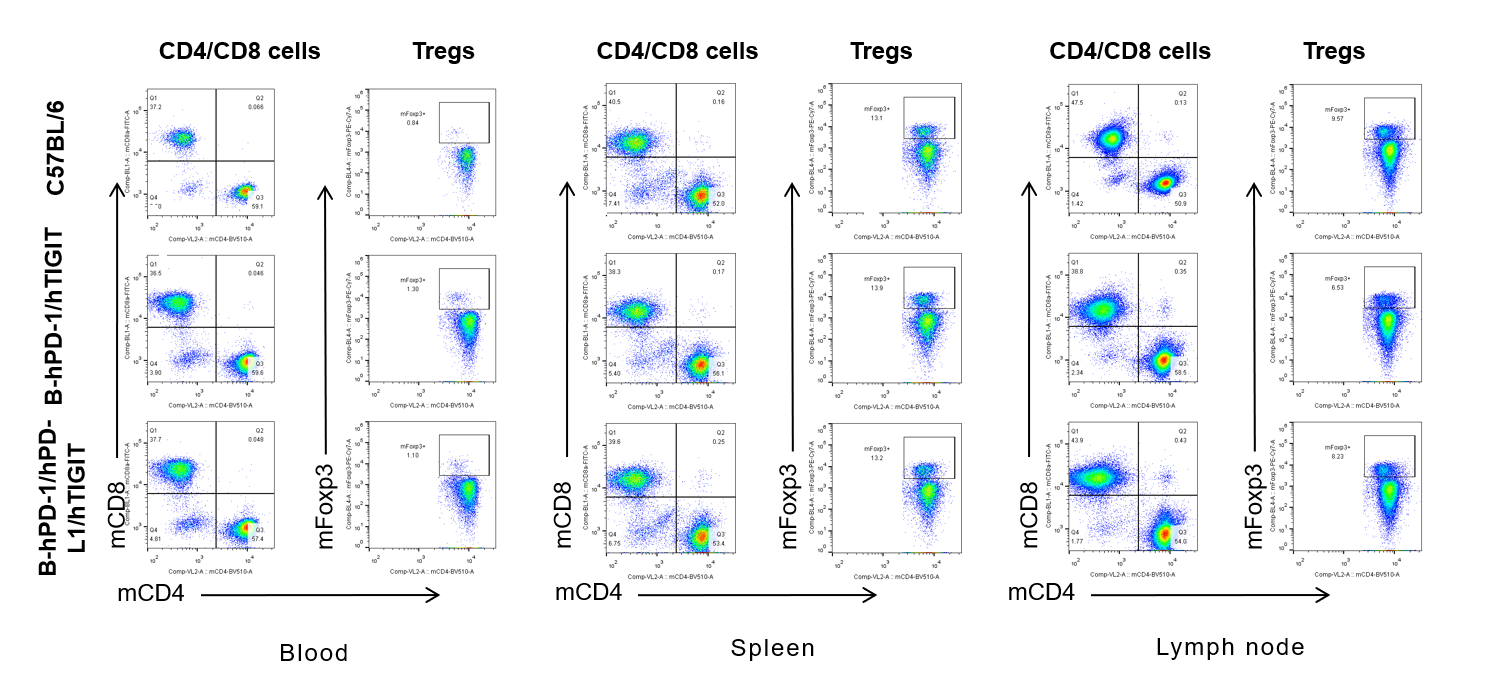
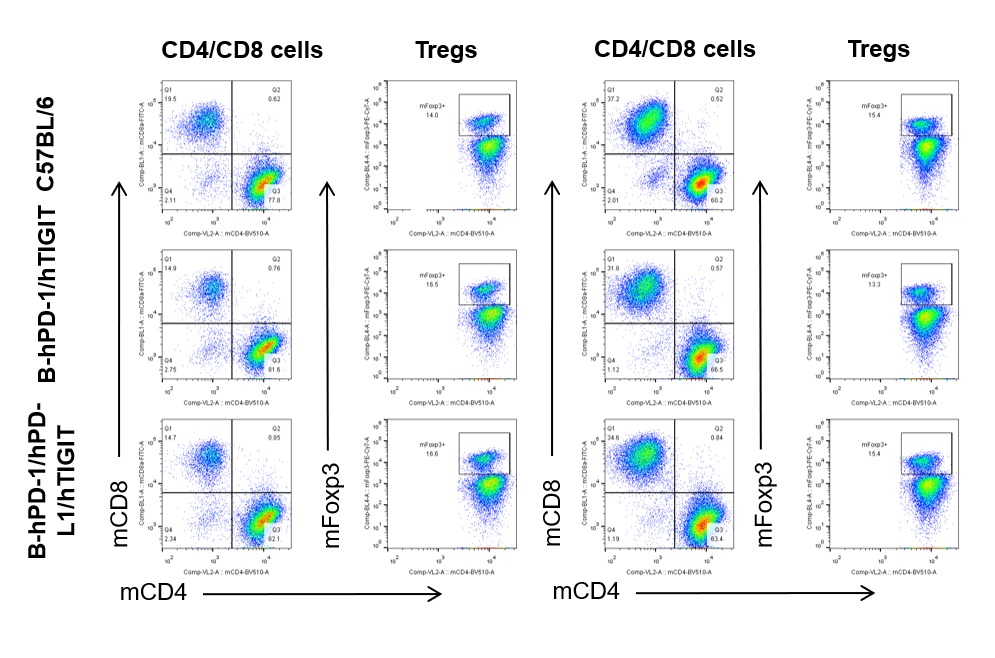
Analysis of blood,spleen and lymph node T cell subpopulations by FACS
Blood, spleen and lymph node leukocytes cell were isolated from female mice in the panel(n=3, 6 week-old). Flow cytometry analysis was performed to assess leukocyte subpopulations. Percent of CD4+T, CD8+T and Tre cells in homozygous B-hPD-1/hPD-L1/hTIGIT mice were similar to those in the C57BL/6 mice and B-hPD-1/hTIGIT mice both at rest and in stimulated with anti-CD3ε in vivo, demonstrating that the humanized mouse does not change the overall development, differentiation or distribution of these cell types in blood, spleen and lymph node.
-
Blood chemistry

-
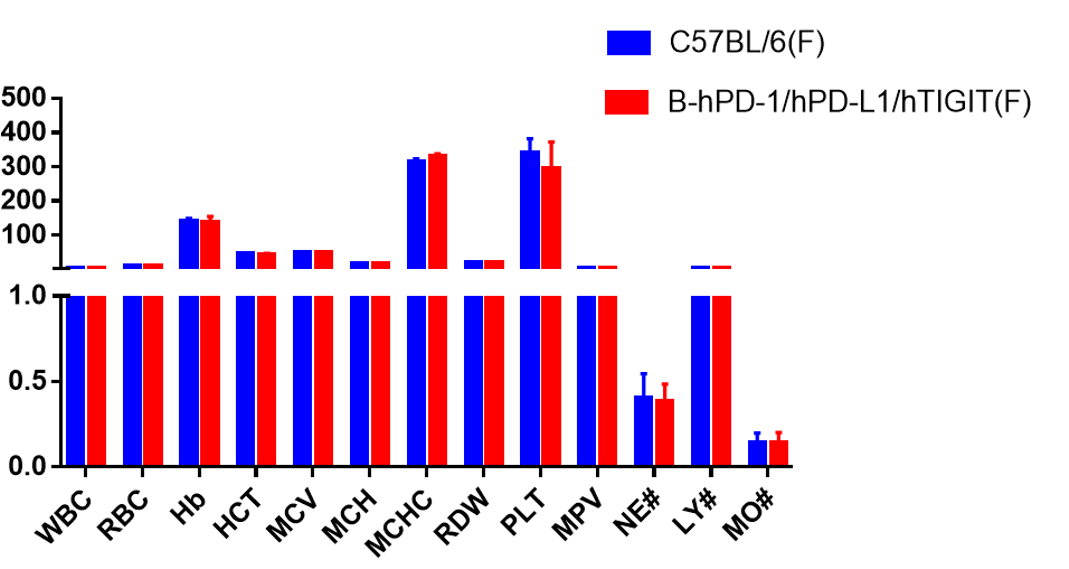
Complete blood count (CBC). Blood from C57BL/6, B-hPD-1/hPD-L1/hTIGIT mice (n=5, 6 week-old, female) ) were collected and analyzed for CBC. Any measurement of B-hPD-1/hPD-L1/hTIGIT mice in the panel were similar to C57BL/6, and there was no differences between male and female mice, indicating that humanized mouse does not change blood cell composition and morphology. Values are expressed as mean ± SEM.
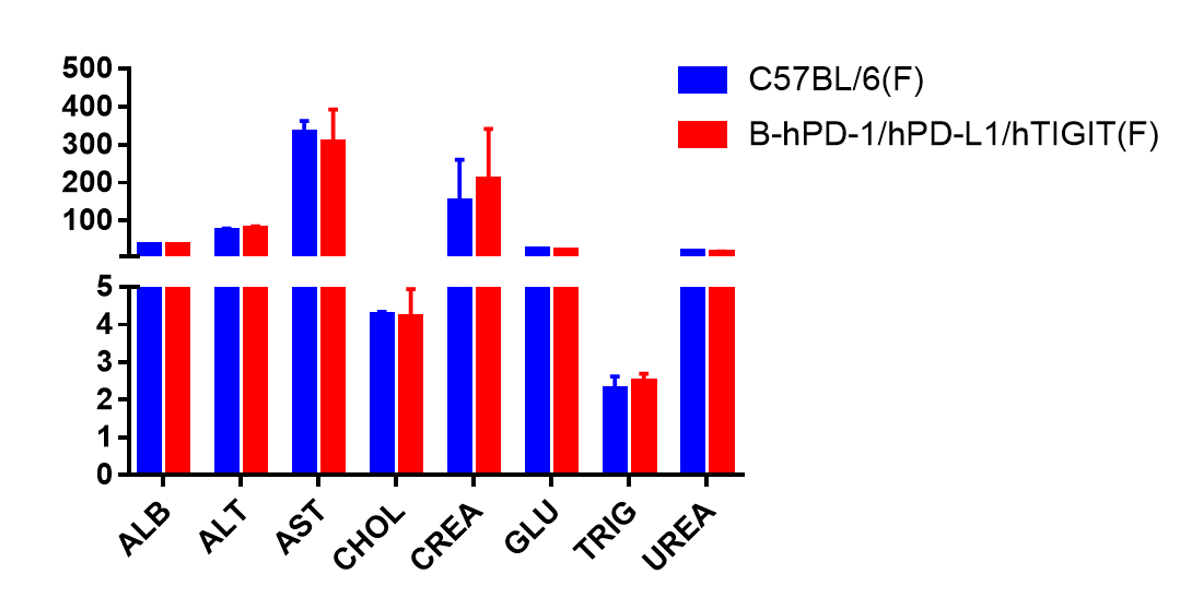
Blood chemistry tests of B-hPD-1/hPD-L1/hTIGIT mice. Serum from C57BL/6, B-hPD-1/hPD-L1/hTIGTI mice (n=5, 6 week-old, female) were collected and analyzed for levels of ALT, AST and other indicators in the panel. There was no differences on either measurement between C57BL/6 and humanized mouse, indicating that humanized mouse does not change ALT and AST levels or health of liver. Values are expressed as mean ± SEM.
-
Combination therapy

-
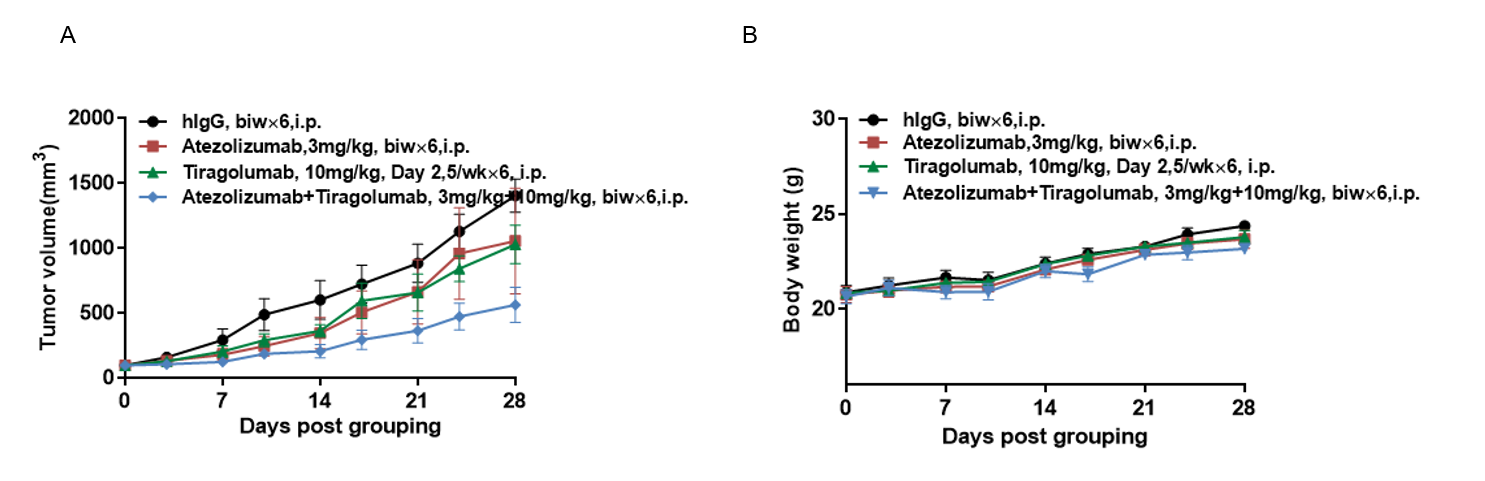
Antitumor activity of of anti-human anti-human PD-L1 Atezolizumab (in house) combined with anti-human Triagolizumab(in house) in B-hPD-1/hPD-L1/hTIGIT mice. (A) anti-human PD-L1 Atezolizumab (in house) combined with anti-human Triagolizumab(in house) inhibited MC38-hPD-L1 tumor growth in B-hPD-1/hPD-L1/hTIGIT mice. Murine colon cancer MC38-hPD-L1 cells (5×105) were subcutaneously implanted into homozygous B-hPD-1/hPD-L1/hTIGIT mice(female, 9 week-old, n=5). Mice were grouped when tumor volume reached approximately 100-150 mm3, at which time they were treated anti-human PD-L1 Atezolizumab (in house) combined with anti-human Triagolizumab(in house) with doses and schedules indicated in panel . (B) Body weight changes durin g treatment. As shown in panel A, combination of anti-hPD-L1 and anti-hTIGIT antibody shows more inhibitory effects than individual groups, demonstrating that the B-hPD-1/hPD-L1/hTIGIT mice provide a powerful preclinical model for in vivo evaluating combination therapy efficacy of hTIGIT antibodies and hPD-L1 antibodies . Values are expressed as mean ± SEM.


