Basic Information
-
Gene Targeting Strategy

-
Gene targeting strategy for B-hIL36R mice. The chimeric IL1RL2 CDS was inserted after the 5’UTR of mouse Il1rl2 gene in B-hIL36R mice. The insertion disrupts the endogenous murine Il1rl2 gene, resulting in the absence of mouse transcripts.
-
mRNA Expression Analysis

-
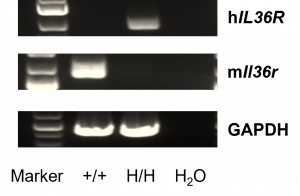
Strain specific analysis of IL36R gene expression in wild type (WT) mice and B-hIL36R mice by RT-PCR. Mouse Il1rl2 mRNA was detectable only in lung of WT mice (+/+). Human IL1RL2 mRNA was detectable only in homozygous B-hIL36R mice (H/H) but not in WT mice (+/+).


Quantitative PCR analysis of IL36R mRNA expression in wild-type (WT) mice and B-hIL36R mice. Total RNA was isolated from heart, liver, spleen, lung, kidney, brain, thymus, lymph node, uterus, ovary, testis, thyroid, skin, muscle, esophagus and small intestine of WT mice and homozygous B-hIL36R mice (n=3, 8 week-old), qPCR method was used to detect the RNA expression of IL36R. The results showed that the abundance of IL36R mRNA in spleen, lung, brain and thymus of B-hIL36R mice were significantly lower than that in WT mice, and the expression level of IL36R mRNA in other tissues of B-hIL36R mice were slightly lower than that in wild mice. The mean and standard deviation from triplicate samples are indicated.
-
Immune Cell Analysis

-
Analysis of spleen leukocytes cell subpopulations in B-hIL36R mice


Analysis of spleen leukocyte subpopulations by FACS. Splenocytes were isolated from female C57BL/6 and B-hIL36R mice (n=3, 7 week-old). Flow cytometry analysis of the splenocytes was performed to assess leukocyte subpopulations. (A) Representative FACS plots. Single live cells were gated for CD45 population and used for further analysis as indicated here. (B) Results of FACS analysis. Percent of T cells, B cells, NK cells, dendritic cells, granulocytes, monocytes and macrophages in homozygous B-hIL36R mice were similar to those in the C57BL/6 mice, demonstrating that introduction of hIL36R in place of its mouse counterpart does not change the overall development, differentiation or distribution of these cell types in spleen. Values are expressed as mean ± SEM.
Analysis of spleen T cell subpopulations in B-hIL36R mice
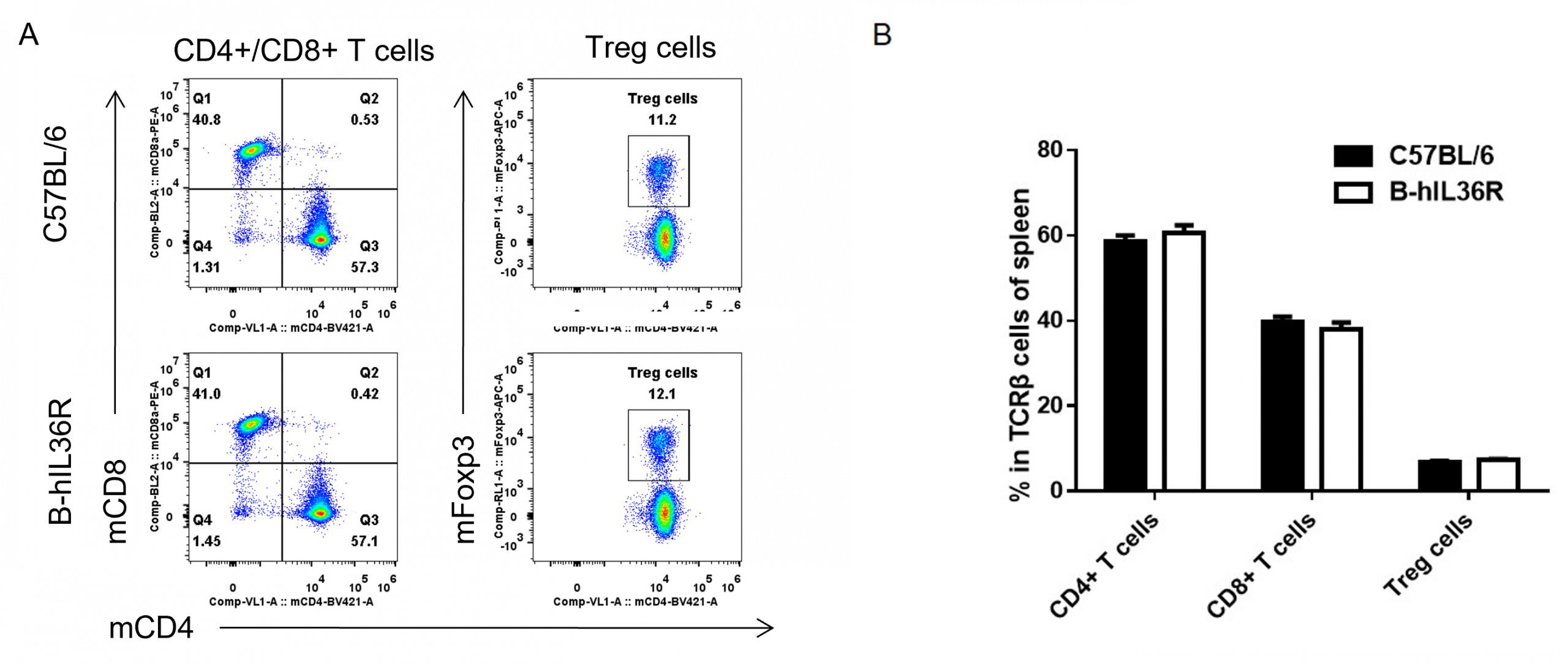
Analysis of spleen T cell subpopulations by FACS. Splenocytes were isolated from female C57BL/6 and B-hIL36R mice (n=3, 7 week-old). Flow cytometry analysis of the splenocytes was performed to assess leukocyte subpopulations. (A) Representative FACS plots. Single live CD45+ cells were gated for CD3 T cell population and used for further analysis as indicated here. (B) Results of FACS analysis. Percent of CD8+ T cells, CD4+ T cells and Treg cells in homozygous B-hIL36R mice were similar to those in the C57BL/6 mice, demonstrating that introduction of hIL36R in place of its mouse counterpart does not change the overall development, differentiation or distribution of these T cell sub types in spleen. Values are expressed as mean ± SEM.
Analysis of lymph node leukocytes cell subpopulations in B-hIL36R mice

Analysis of lymph node leukocyte subpopulations by FACS. Leukocytes were isolated from female C57BL/6 and B-hIL36R mice (n=3, 7 week-old). Flow cytometry analysis of the leukocytes was performed to assess leukocyte subpopulations. (A) Representative FACS plots. Single live cells were gated for CD45 population and used for further analysis as indicated here. (B) Results of FACS analysis. Percent of T cells, B cells and NK cells in homozygous B-hIL36R mice were similar to those in the C57BL/6 mice, demonstrating that introduction of hIL36R in place of its mouse counterpart does not change the overall development, differentiation or distribution of these cell types in lymph node. Values are expressed as mean ± SEM.
Analysis of lymph node T cell subpopulations in B-hIL36R mice

Analysis of lymph node T cell subpopulations by FACS. Leukocytes were isolated from female C57BL/6 and B-hIL36R mice (n=3, 7 week-old). Flow cytometry analysis of the leukocytes was performed to assess leukocyte subpopulations. (A) Representative FACS plots. Single live CD45+ cells were gated for CD3 T cell population and used for further analysis as indicated here. (B) Results of FACS analysis. Percent of CD8+ T cells, CD4+ T cells and Treg cells in homozygous B-hIL36R mice were similar to those in the C57BL/6 mice, demonstrating that introduction of hIL36R in place of its mouse counterpart does not change the overall development, differentiation or distribution of these T cell sub types in lymph node. Values are expressed as mean ± SEM.
Analysis of blood leukocytes cell subpopulations in B-hIL36R mice


Analysis of blood leukocyte subpopulations by FACS. Blood cells were isolated from female C57BL/6 and B-hIL36R mice (n=3, 7 week-old). Flow cytometry analysis of the blood leukocytes was performed to assess leukocyte subpopulations. (A) Representative FACS plots. Single live cells were gated for CD45 population and used for further analysis as indicated here. (B) Results of FACS analysis. Percent of T cells, B cells, NK cells, dendritic cells, granulocytes, monocytes and macrophages in homozygous B-hIL36R mice were similar to those in the C57BL/6 mice, demonstrating that introduction of hIL36R in place of its mouse counterpart does not change the overall development, differentiation or distribution of these cell types in blood. Values are expressed as mean ± SEM.
Analysis of blood T cell subpopulations in B-hIL36R mice

Analysis of blood T cell subpopulations by FACS. Blood cells were isolated from female C57BL/6 and B-hIL36R mice (n=3, 7 week-old). Flow cytometry analysis of the leukocytes was performed to assess leukocyte subpopulations. (A) Representative FACS plots. Single live CD45+ cells were gated for CD3 T cell population and used for further analysis as indicated here. (B) Results of FACS analysis. Percent of CD8+ T cells, CD4+ T cells and Treg cells in homozygous B-hIL36R mice were similar to those in the C57BL/6 mice, demonstrating that introduction of hIL36R in place of its mouse counterpart does not change the overall development, differentiation or distribution of these T cell sub types in blood. Values are expressed as mean ± SEM.
-
In vivo Efficacy of an Anti-Human IL36R Antibody in a Psoriasis Model

-
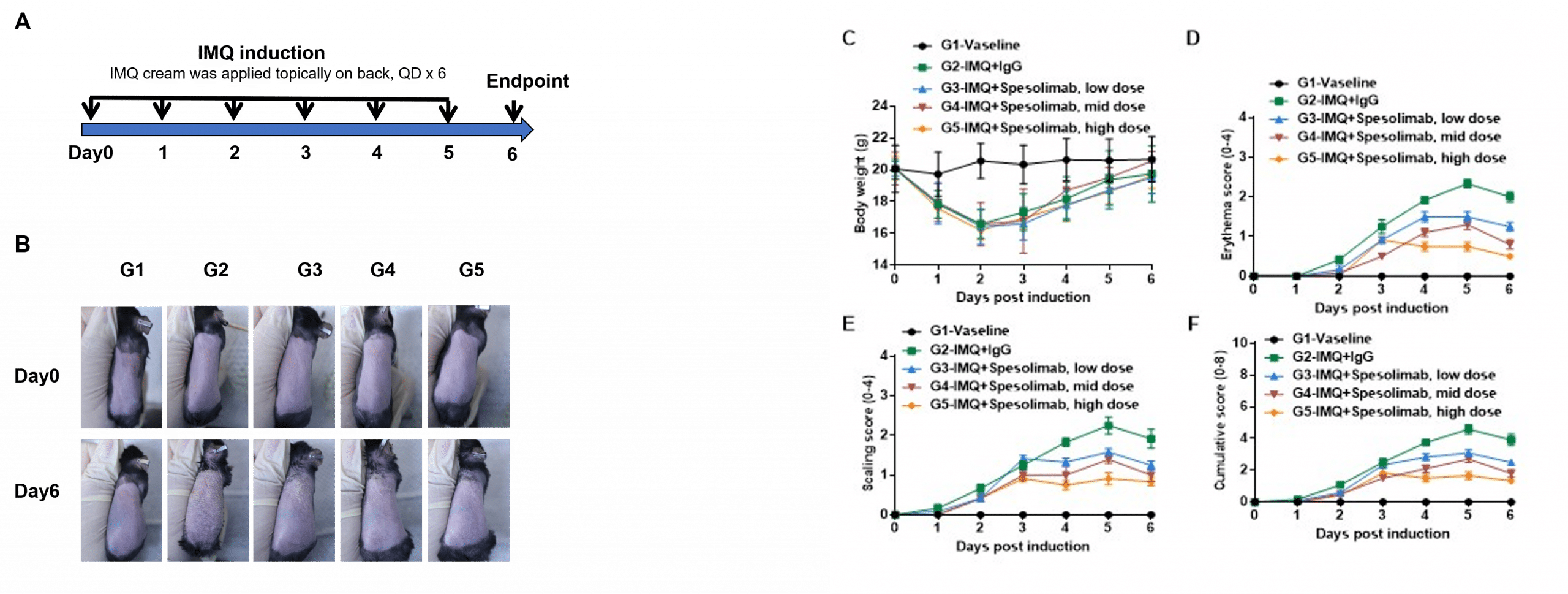
IMQ-induced skin inflammation in B-hIL36R mice phenotypically resembles psoriasis. Humanized B-hIL36R mice (female, 9 week-old, n=6) were scored daily for up to 7 days for body weight and clinical signs of skin inflammation following treatment with imiquimod (IMQ) cream. Mice in each group were treated with different doses of Spesolimab (in-house). (A) Experimental schedule for induction of psoriasis-like skin lesions in B-hIL36R mice. (B) Phenotypic presentation of mouse back skin at day 0 and day 6. (C) Body weight changes during treatment. (D-E) Erythema, scaling and cumulative (erythema plus scaling) score of the back was scored daily. Values are expressed as mean ± SEM.
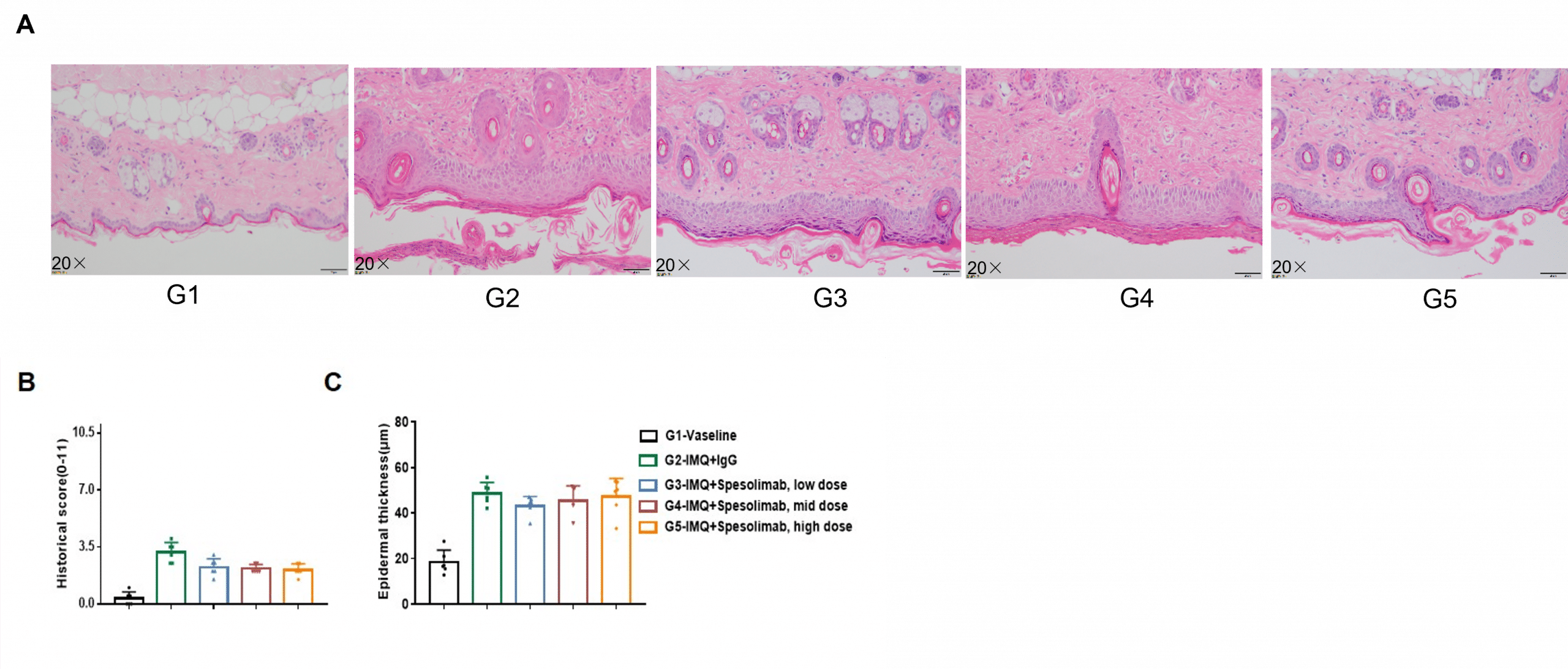
Dose-dependent effects of anti-human IL36R antibodies on keratinocyte proliferation and inflammatory cell infiltration in IMQ induced psoriasis-like skin lesions in B-hIL36R mice. Back skin was collected at the endpoint and stained with hematoxylin and eosin (H&E). (A) H&E staining of the back skin. (B) Histological changes, and (C) epidermal thickness of the mice were scored. Results indicated that therapeutic effects of Spesolimab (in-house) on psoriasis-like skin lesions in B-hIL36R mice were dose-dependent, confirming that B-hIL36R mice provide a powerful model for in vivo evaluation of anti-human IL36R antibodies. Values are expressed as mean ± SEM.
-
Poster



