Basic Information
-
Protein expression analysis

-
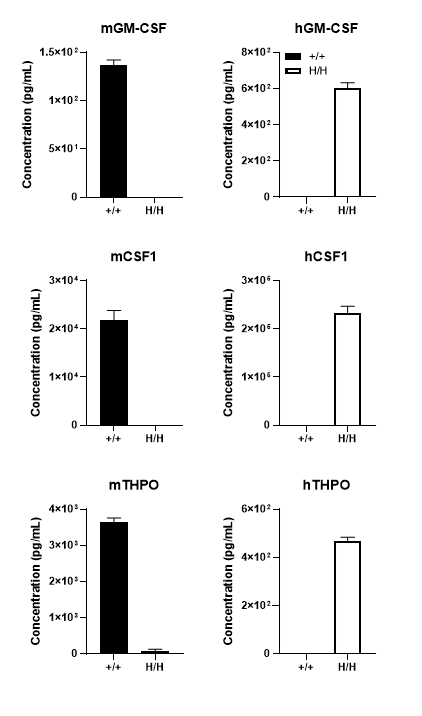
Species-specific GM-CSF, CSF1 and THPO protein expression analysis in wild-type B-NDG and B-NDG MGMT3 mice. Following LPS stimulation in vivo, serum was collected from wild-type B-NDG (+/+) and homozygous B-NDG MGMT3 (H/H) mice and analyzed by ELISA (n=3) using species-specific kits. Murine GM-CSF, CSF1 and THPO proteins were detected in B-NDG mice, while human GM-CSF, CSF1 and THPO proteins were detected in B-NDG MGMT3 mice. Mouse and/or human IL3 protein expression was not detected in immunodeficient B-NDG and/or B-NDG MGMT3 mice due to the lack of mature (activated) T cells.
-
Analysis of leukocyte subpopulation in spleen

-
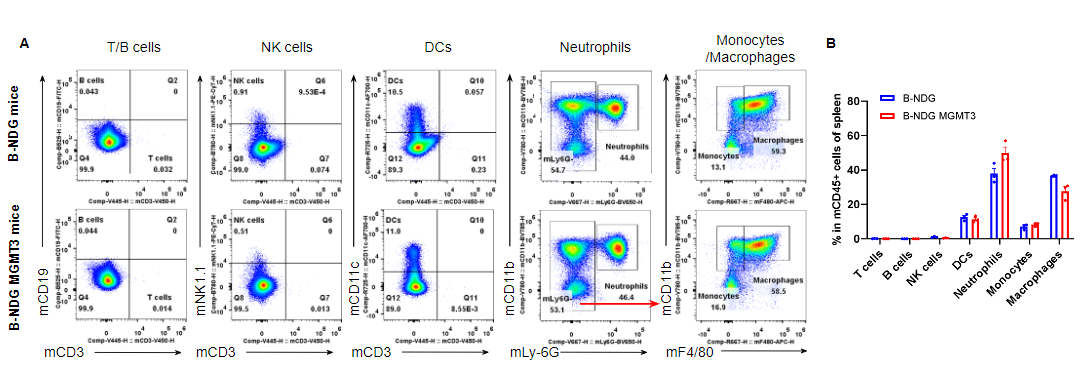
Analysis of leukocyte subpopulations in spleen by flow cytometry. Splenocytes were isolated from male B-NDG and B-NDG MGMT3 mice (n=3, 7-8-week-old). Flow cytometry analysis of the splenocytes was performed to assess leukocyte subpopulations. A. Representative FACS plots. Single live cells were gated for the CD45+ population and used for further analysis as indicated here. B. Results of FACS analysis. Percentages of T cells, B cells, NK cells, dendritic cells, neutrophils, monocytes and macrophages in homozygous B-NDG MGMT3 mice were similar to those in the B-NDG mice, demonstrating that IL3, GM-CSF, CSF1 and THPO humanized does not change the overall development, differentiation or distribution of these cell types in spleen. Values are expressed as mean ± SEM.
-
Analysis of leukocyte subpopulation in blood

-
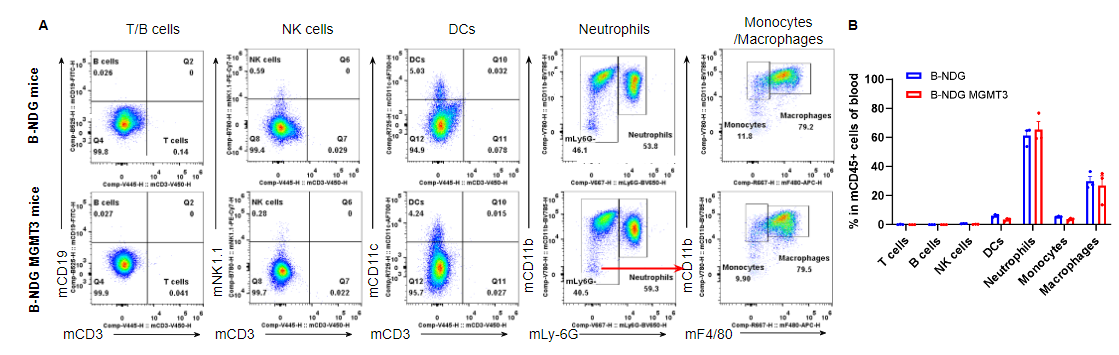
Analysis of leukocyte subpopulations in blood by flow cytometry. Blood was isolated from male B-NDG and B-NDG MGMT3 mice (n=3, 7-8-week-old). Flow cytometry analysis of the blood cells was performed to assess leukocyte subpopulations. A. Representative FACS plots. Single live cells were gated for the CD45+ population and used for further analysis as indicated here. B. Results of FACS analysis. Percentages of T cells, B cells, NK cells, dendritic cells, neutrophils, monocytes and macrophages in homozygous B-NDG MGMT3 mice were similar to those in the B-NDG mice, demonstrating that IL3, GM-CSF, CSF1 and THPO humanized does not change the overall development, differentiation or distribution of these cell types in blood. Values are expressed as mean ± SEM.
-
Analysis of leukocyte subpopulation in bone marrow

-
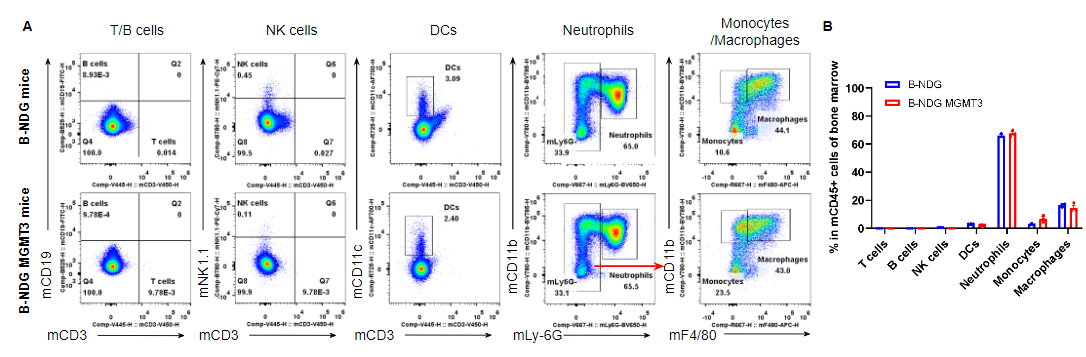
Analysis of leukocyte subpopulations in bone marrow by flow cytometry. Bone marrow was isolated from male B-NDG and B-NDG MGMT3 mice (n=3, 7-8-week-old). Flow cytometry analysis of the cells from bone marrow was performed to assess leukocyte subpopulations. A. Representative FACS plots. Single live cells were gated for the CD45+ population and used for further analysis as indicated here. B. Results of FACS analysis. Percent of T cells, B cells, NK cells, dendritic cells, neutrophils, monocytes and macrophages in homozygous B-NDG MGMT3 mice were similar to those in the B-NDG mice, demonstrating that IL3, GM-CSF, CSF1 and THPO humanized does not change the overall development, differentiation or distribution of these cell types in bone marrow. Values are expressed as mean ± SEM.
-
Human CD34+ HSC engraftment for human immune system reconstitution

-
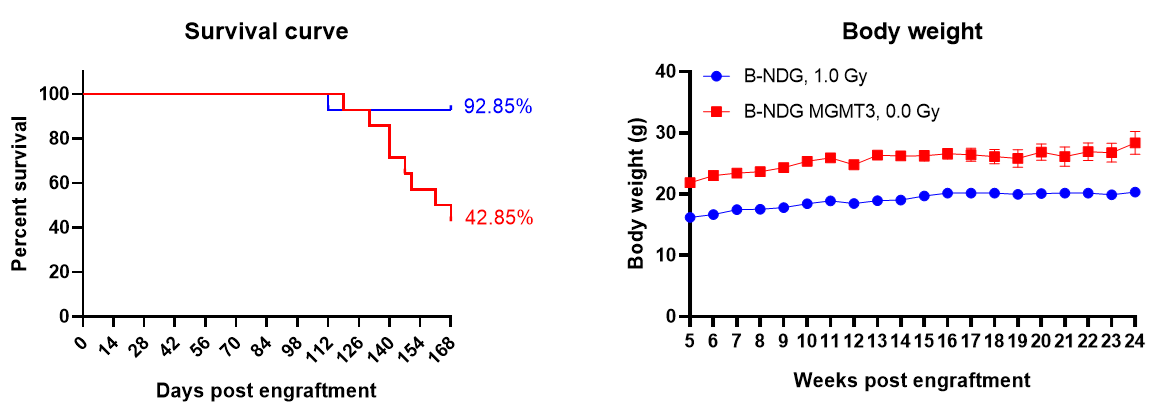
Human CD34+ HSC engraftment for human immune system reconstitution. Wild-type B-NDG and B-NDG MGMT3 (both sex, 24-72 hr after birth, n=15) mice were engrafted with human CD34+ HSCs (3E4) by intravenous (temporal vein) injection. B-NDG mice were treated with 1.0 Gy-irradiation, while B-NDG MGMT3 mice were not irradiated. (A) Survival rates were analyzed by Kaplan Meier survival curves. (B) Body weight measurements. Results showed that the survival rate of B-NDG MGMT3 mice were similar to that of B-NDG mice until 16 weeks post human CD34+ HSC engraftment, while body weight of B-NDG MGMT3 mice was significantly higher than that of B-NDG mice. Values are expressed as mean ± SEM. HSCs: hematopoietic stem cells.
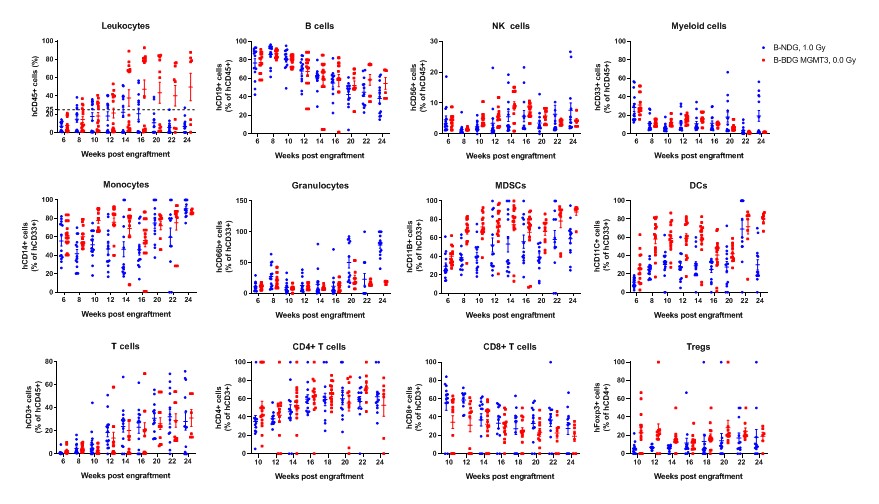
Human CD34+ HSCs (3E4) were intravenous (temporal vein) engrafted into wild-type B-NDG mice and homozygous B-NDG MGMT3 mice (both sex, 24-72 hr after birth, n=15). B-NDG mice were treated with 1.0 Gy-irradiation. B-NDG MGMT3 mice were not irradiated. Peripheral blood lymphocytes from the two mice after engraftment with human CD34+ HSCs were analyzed with flow cytometry. Results showed that the proportion of CD45+ cells in B-NDG MGMT3 mice reached 25% starting from 12 weeks after engraftment and continued to rise, significantly higher than that in B-NDG mice. The proportions of monocytes, MDSCs, DCs and Tregs in B-NDG MGMT3 mice were higher than that in B-NDG mice. Values are expressed as mean ± SEM.
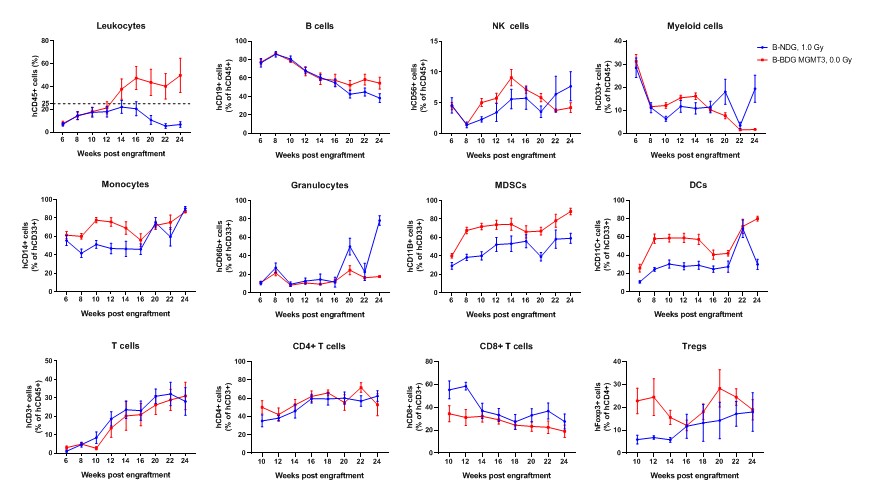
Human CD34+ HSCs (3E4) were intravenous (temporal vein) engrafted into wild-type B-NDG mice and homozygous B-NDG MGMT3 mice (both sex, 24-72 hr after birth, n=15). B-NDG mice were treated with 1.0 Gy-irradiation. B-NDG MGMT3 mice were not irradiated. Peripheral blood lymphocytes from the two mice after engraftment with human CD34+ HSCs were analyzed with flow cytometry. Results showed that the proportion of CD45+ cells in B-NDG MGMT3 mice reached 25% starting from 12 weeks after engraftment and continued to rise, significantly higher than that in B-NDG mice. The proportions of monocytes, MDSCs, DCs and Tregs in B-NDG MGMT3 mice were higher than that in B-NDG mice. Values are expressed as mean ± SEM.
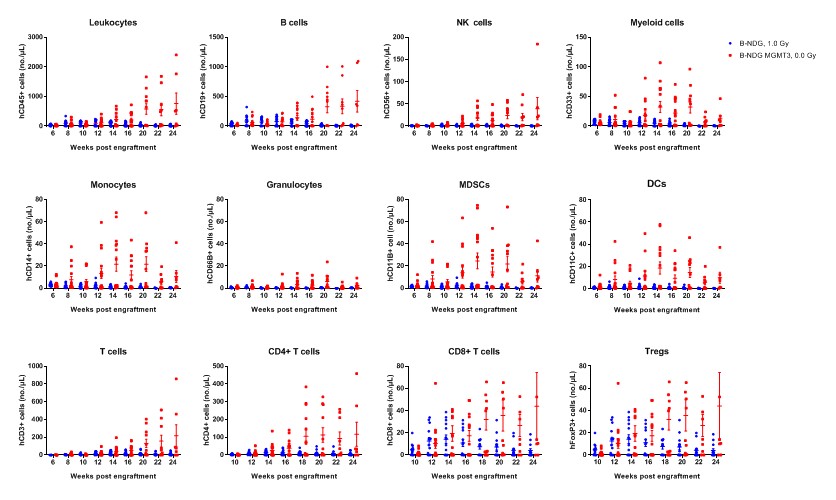
Human CD34+ HSCs (3E4) were intravenous (temporal vein) engrafted into wild-type B-NDG mice and homozygous B-NDG MGMT3 mice (both sex, 24-72 hr after birth, n=15). B-NDG mice were treated with 1.0 Gy-irradiation. B-NDG MGMT3 mice were not irradiated. Peripheral blood lymphocytes from the two mice after engraftment with human CD34+ HSCs were analyzed with flow cytometry. Results showed that the cell numbers of all the cells analyzed from 12 weeks after engraftment in B-NDG MGMT3 mice were higher than that in B-NDG mice. Values are expressed as mean ± SEM. Values are expressed as mean ± SEM.
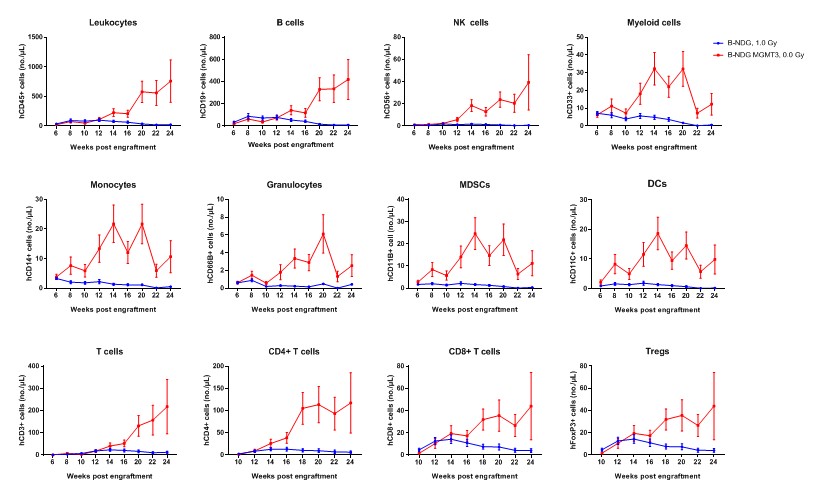
Human CD34+ HSCs (3E4) were intravenous (temporal vein) engrafted into wild-type B-NDG mice and homozygous B-NDG MGMT3 mice (both sex, 24-72 hr after birth, n=15). B-NDG mice were treated with 1.0 Gy-irradiation. B-NDG MGMT3 mice were not irradiated. Peripheral blood lymphocytes from the two mice after engraftment with human CD34+ HSCs were analyzed with flow cytometry. Results showed that the cell numbers of all the cells analyzed from 12 weeks after engraftment in B-NDG MGMT3 mice were higher than that in B-NDG mice. Values are expressed as mean ± SEM. Values are expressed as mean ± SEM.
-
Summary

-
Protein expression analysis:
- Mouse GM-CSF, CSF1 and THPO were only detectable in B-NDG mice. Human GM-CSF, CSF1 and THPO were only detectable in B-NDG MGMT3 mice. As IL3 is mainly expressed in activated T cells and there is no mature T cells in B-NDG background mice, mouse or human IL3 was not detectable in both of the two mice.
- Leukocytes cell subpopulation analysis:
- IL3, GM-CSF, CSF1 and THPO humanized does not change the overall development, differentiation or distribution of immune cell types in spleen, blood and bone marrow.
- Human CD34+ HSC engraftment for human immune system reconstitution
- The survival rate of B-NDG MGMT3 mice was similar to that of B-NDG mice until 16 weeks after human CD34+ HSCs engraftment. But the body weight of B-NDG MGMT3 mice was significantly higher than that of B-NDG mice.
- The proportion of CD45+ cells in B-NDG MGMT3 mice reached 25% starting from 12 weeks after engraftment and continued to rise, significantly higher than that in B-NDG mice. The proportions of monocytes, MDSCs, DCs and Tregs in B-NDG MGMT3 mice were higher than that in B-NDG mice.
- The cell numbers of all the cells analyzed from 12 week after engraftment in B-NDG MGMT3 mice were higher than that in B-NDG mice.
-
Poster



