Basic Information
-
Targeting Strategy

-
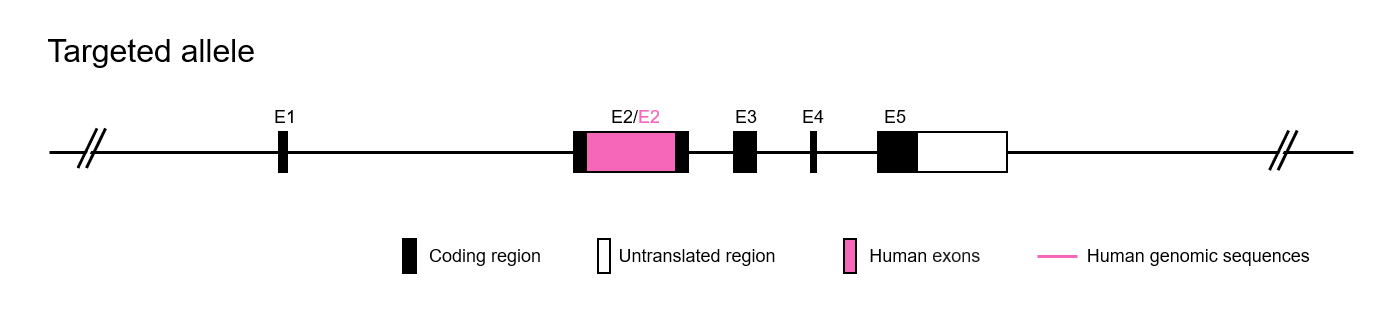
The exon 2 of mouse PD-1 gene that encode the extracellular domain was replaced by human PD-1 exon 2 in B-hPD-1 mice.
-
Details

-
mRNA expression analysis

Strain specific analysis of PD-1 gene expression in WT and B-hPD-1 mice by RT-PCR.
Mouse PD-1 mRNA was detected only in splenocytes of wild-type (+/+) mice. Human PD-1 mRNA was detected only in H/H mice, but not in +/+ mice.Phenotype
Protein Expression Analysis
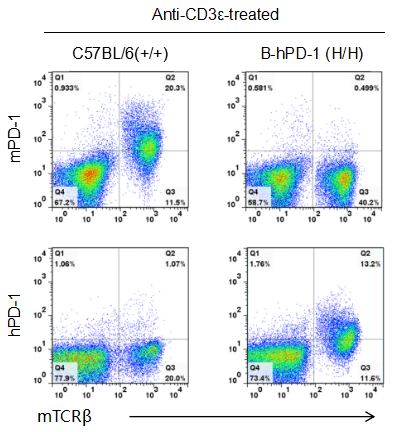
Strain specific PD-1 expression analysis in homozygous B-hPD-1 mice by flow cytometry.
Splenocytes were collected from WT and homozygous B-hPD-1 (H/H) mice stimulated with anti-CD3ε in vivo, and analyzed by flow cytometry with species-specific anti-PD-1 antibody. Mouse PD-1 was detected in WT mice. Human PD-1 was exclusively detected in homozygous B-hPD-1 mice but not WT mice.
Application
In vivo efficacy of anti-human PD-1 antibody
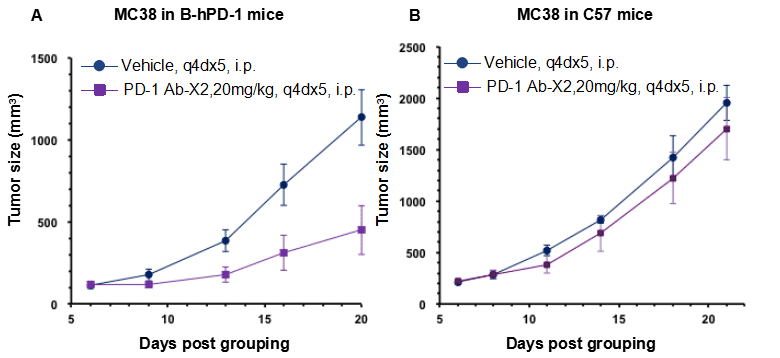
Antitumor activity of anti-human PD-1 antibody in B-hPD-1 mice and C57BL/6 mice.
Anti-human PD-1 antibody inhibited MC38 tumor growth in B-hPD-1 mice. (A) Murine colon cancer MC38 cells (5×105) were subcutaneously implanted into homozygous B-hPD-1 mice (female, 6 week-old, n=6). Mice were grouped when tumor volume reached approximately 100 mm3, at which time they were treated with anti-human PD-1 antibody and schedules indicated in panel A, as shown in panel A, human PD-1 antibody X2 efficacious in controlling tumor growth in the homozygous B-hPD-1 mice but not in the wild type C57BL/6 mice (B) The results demonstrating that B-hPD-1 mice provide a powerful preclinical model for in vivo evaluation of anti-human PD-1 antibodies. Values are expressed as mean ± SEM.
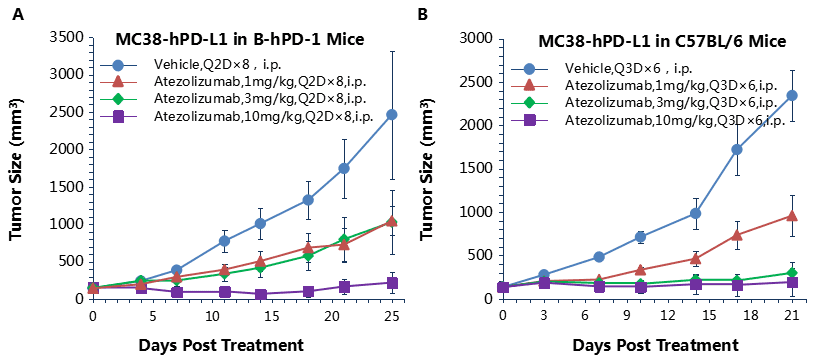
Antitumor activity of anti-human PD-L1 antibody in B-hPD-L1 and C57BL/6 mice.
Anti-human PD-L1 antibody atezolizumab inhibited MC38-hPD-L1 tumor growth in B-hPD-L1 mice (A) and wild type C57BL/6 mice (B). Murine colon cancer MC38-hPD-L1 cells (2×105) were subcutaneously implanted into homozygous B-hPD-1 mice and C57BL/6 (female, 6-8 week-old, n=6). Mice were grouped when tumor volume reached approximately 100 mm3, at which time they were treated with anti-human PD-L1 antibody with doses and schedules indicated in panel , human PD-L1 efficacious in controlling tumor growth in homozygous B-hPD-1 mice and wild type C57BL/6 mice (B). Values are expressed as mean ± SEM.
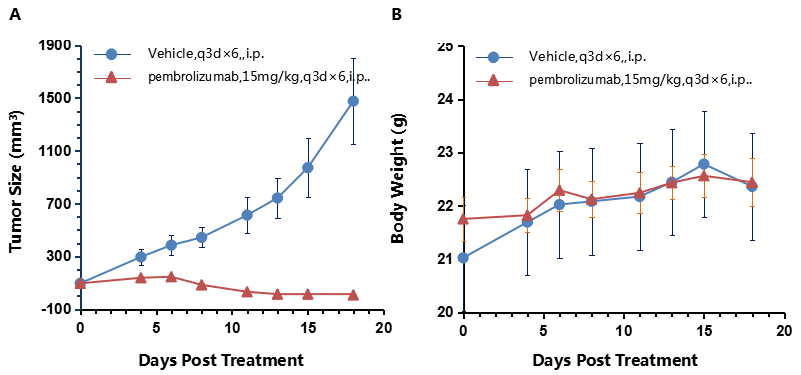
Antitumor activity of anti-human PD-1 antibody (pembrolizumab) in B-hPD-1 mice.
(A) Pembrolizumab inhibited MC38 tumor growth in B-hPD-1 mice. Murine colon cancer MC38 cells (5×105) were subcutaneously implanted into homozygous B-hPD-1 mice (female, 6 week-old, n=10). Mice were grouped when tumor volume reached approximately 100 mm3, at which time they were treated with anti-human PD-1 antibody with doses and schedules indicated in panel A. (B) Body weight changes during treatment. As shown in panel A, anti-human PD-1 antibody was efficacious in controlling tumor growth in B-hPD-1 mice, demonstrating that the B-hPD-1 mice provide a powerful preclinical model for in vivo evaluation of anti-human PD-1 antibodies. Values are expressed as mean ± SEM.
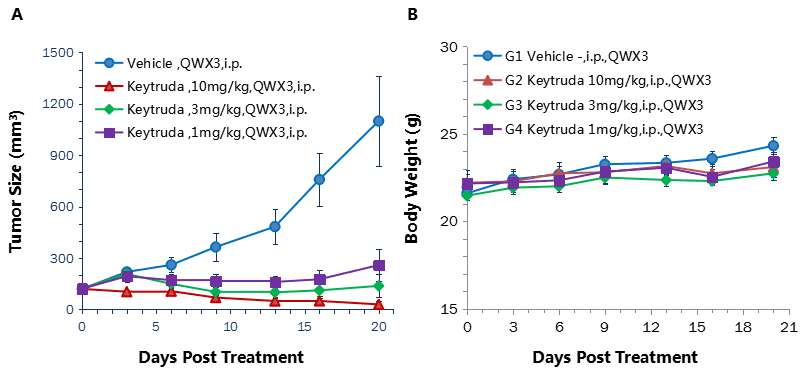
Antitumor activity of anti-human PD-1 antibody in B-hPD-1 mice.
(A) Anti-human PD-1 antibody inhibited MC38 tumor growth in B-hPD-1 mice. Murine colon cancer MC38 cells (5×105) were subcutaneously implanted into homozygous B-hPD-1 mice (female, 4-6 week-old, n=5). Mice were grouped when tumor volume reached approximately 100 mm3, at which time they were treated with three anti-human PD-1 antibodies with doses and schedules indicated in panel. (B) Body weight changes during treatment. As shown in panel A, anti-human PD-1 antibodies were efficacious in controlling tumor growth in B-hPD-1 mice, demonstrating that the B-hPD-1 mice provide a powerful preclinical model for in vivo evaluation of anti-human PD-1 antibodies. Values are expressed as mean ± SEM.
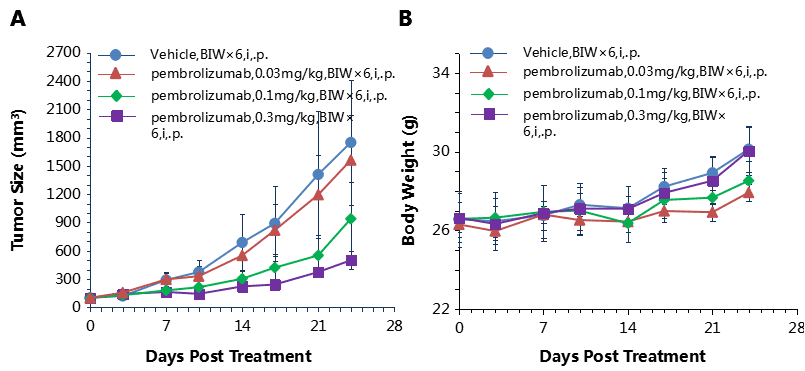
Antitumor activity of anti-human PD-1 antibody in B-hPD-1 mice.
(A) Anti-human PD-1 antibody inhibited MC38 tumor growth in B-hPD-1 mice. Murine colon cancer MC38 cells (5×105) were subcutaneously implanted into homozygous B-hPD-1 mice (male, 4-6 week-old, n=5). Mice were grouped when tumor volume reached approximately 100 mm3, at which time they were treated with anti-human PD-1 antibody with doses and schedules indicated in panel (A). (B) Body weight changes during treatment. As shown in panel A, anti-human PD-1 antibody was efficacious in controlling tumor growth in B-hPD-1 mice, demonstrating that B-hPD-1 mice provide a powerful preclinical model for in vivo evaluation of anti-human PD-1 antibodies. Values are expressed as mean ± SEM.
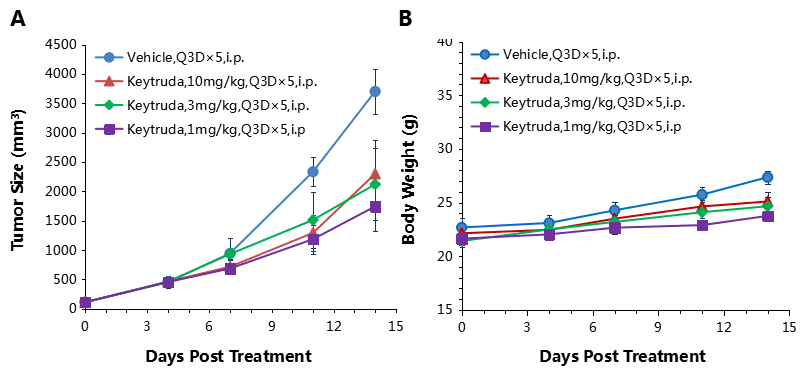
Antitumor activity of anti-human PD-1 antibody in B-hPD-1 mice.
(A) Anti-human PD-1 antibody inhibited EL4 tumor growth in B-hPD-1 mice. Murine lymphoma cancer EL4 cells were subcutaneously implanted into homozygous B-hPD-1 mice (n=5). Mice were grouped when tumor volume reached approximately 150±50 mm3, at which time they were treated with three anti-human PD-1 antibodies with doses and schedules indicated in panel. (B) Body weight changes during treatment. As shown in panel A, anti-human PD-1 antibodies were efficacious in controlling tumor growth in B-hPD-1 mice, demonstrating that the B-hPD-1 mice provide a powerful preclinical model for in vivo evaluation of anti-human PD-1 antibodies. Values are expressed as mean ± SEM.
In vivo efficacy of anti human PD-L1 antibody
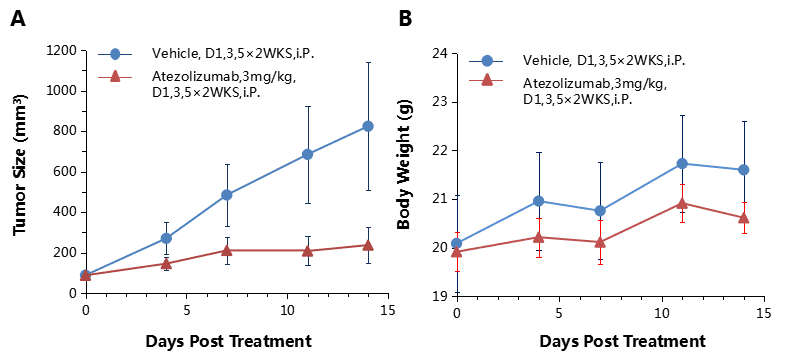
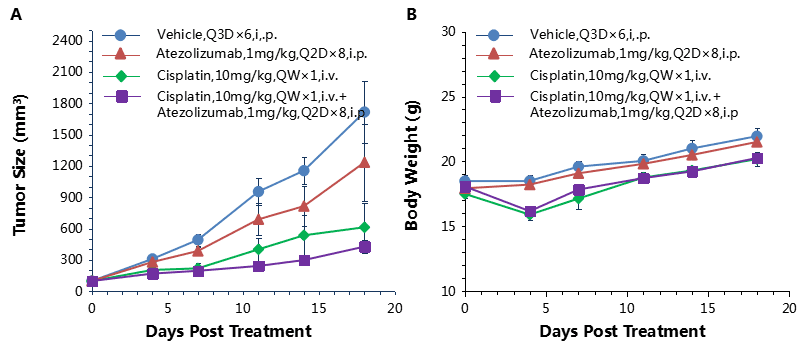
Combination therapy of anti-human PD-1 Ab and chemotherapy drug
Antitumor activity of anti-human PD-1 antibody combined with cisplatin in B-hPD-1 mice.
(A) Anti-human PD-1 antibody combined with cisplatin inhibited MC38-hPD-L1 tumor growth in B-hPD-1 mice. Murine colon cancer MC38-hPD-L1 cells (5×105) were subcutaneously implanted into homozygous B-hPD-1 mice (female, 5-8 week-old, n=8). Mice were grouped when tumor volume reached approximately 150±50 mm3, at which time they were treated with anti-human PD-1 antibodies and cisplatin with doses and schedules indicated in panel. (B) Body weight changes during treatment. As shown in panel A, combination of anti-hPD-1 antibody and the chemotherapy drug cisplatin shows more efficaciously inhibitory effects than individual groups, demonstrating that the B-hPD-1 mice provide a powerful preclinical model for in vivo evaluating combination therapy efficacy of hPD-1 antibodies and chemotherapy drugs. Values are expressed as mean ± SEM.
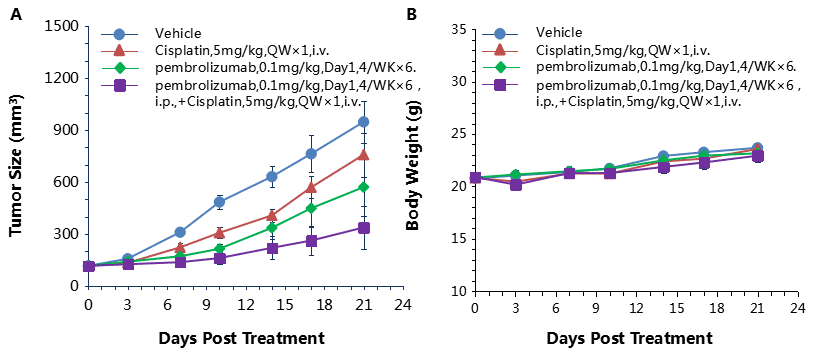
Combination therapy of anti-human PD-1 Ab and chemotherapy drug
Antitumor activity of anti-human PD-L1 antibody combined with cisplatin in B-hPD-1 mice.
(A) Anti-human PD-L1 antibody combined with cisplatin inhibited MC38-hPD-L1 tumor growth in B-hPD-1 mice. Murine colon cancer MC38-hPD-L1 cells (5×105) were subcutaneously implanted into homozygous B-hPD-1 mice (female, 5-8 week-old, n=8). Mice were grouped when tumor volume reached approximately 150±50 mm3, at which time they were treated with anti-human PD-L1 antibody and cisplatin with doses and schedules indicated in panel. (B) Body weight changes during treatment. As shown in panel A, combination of anti-hPD-L1 antibody and the chemotherapy drug cisplatin shows more efficaciously inhibitory effects than individual groups, demonstrating that the B-hPD-1 mice provide a powerful preclinical model for in vivo evaluating combination therapy efficacy of hPD-L1 antibodies and chemotherapy drugs. Values are expressed as mean ± SEM.
-
Publications using B-hPD-1 mice

-
- Huang J, Liu D, Wang Y, Liu L, Li J, Yuan J, Jiang Z, Jiang Z, Hsiao WW, Liu H, Khan I, Xie Y, Wu J, Xie Y, Zhang Y, Fu Y, Liao J, Wang W, Lai H, Shi A, Cai J, Luo L, Li R, Yao X, Fan X, Wu Q, Liu Z, Yan P, Lu J, Yang M, Wang L, Cao Y, Wei H, Leung EL. Ginseng polysaccharides alter the gut microbiota and kynurenine/tryptophan ratio, potentiating the antitumour effect of antiprogrammed cell death 1/programmed cell death ligand 1 (anti-PD-1/PD-L1) immunotherapy. Gut. 2021 May 18:gutjnl-2020-321031. doi: 10.1136/gutjnl-2020-321031. Epub ahead of print. PMID: 34006584.
- Hong Y, Feng Y, Sun H, Zhang B, Wu H, Zhu Q, Li Y, Zhang T, Zhang Y, Cui X, Li Z, Song X, Li K, Liu M, Liu Y. Tislelizumab uniquely binds to the CC’ loop of PD-1 with slow-dissociated rate and complete PD-L1 blockage. FEBS Open Bio. 2021 Mar;11(3):782-792. doi: 10.1002/2211-5463.13102. Epub 2021 Feb 16. PMID: 33527708; PMCID: PMC7931243.
-
References

-
- Nat Commun.2017 Feb 6;8:14369. doi: 10.1038/ncomms14369.
- EMBO J.1992 Nov;11(11):3887-95.
- J Exp Med.2000 Oct 2;192(7):1027-34.
- 2001 Jan 12;291(5502):319-22.


