Basic Information
-
Targeting Strategy

-
Gene targeting strategy for B-hPD-L1/h4-1BB mice. The exon 3 of mouse Pdl1 gene that encode the IgV domain were replaced by human PD-L1 exon 3 in B-hPD-L1/h4-1BB mice. The exon 2-7 of mouse 4-1BB gene that encode the extracellular domain were replaced by human 4-1BB exon 2-7 in B-hPD-L1/h4-1BB mice.
-
Details

-
Phenotype
Protein Expression Analysis
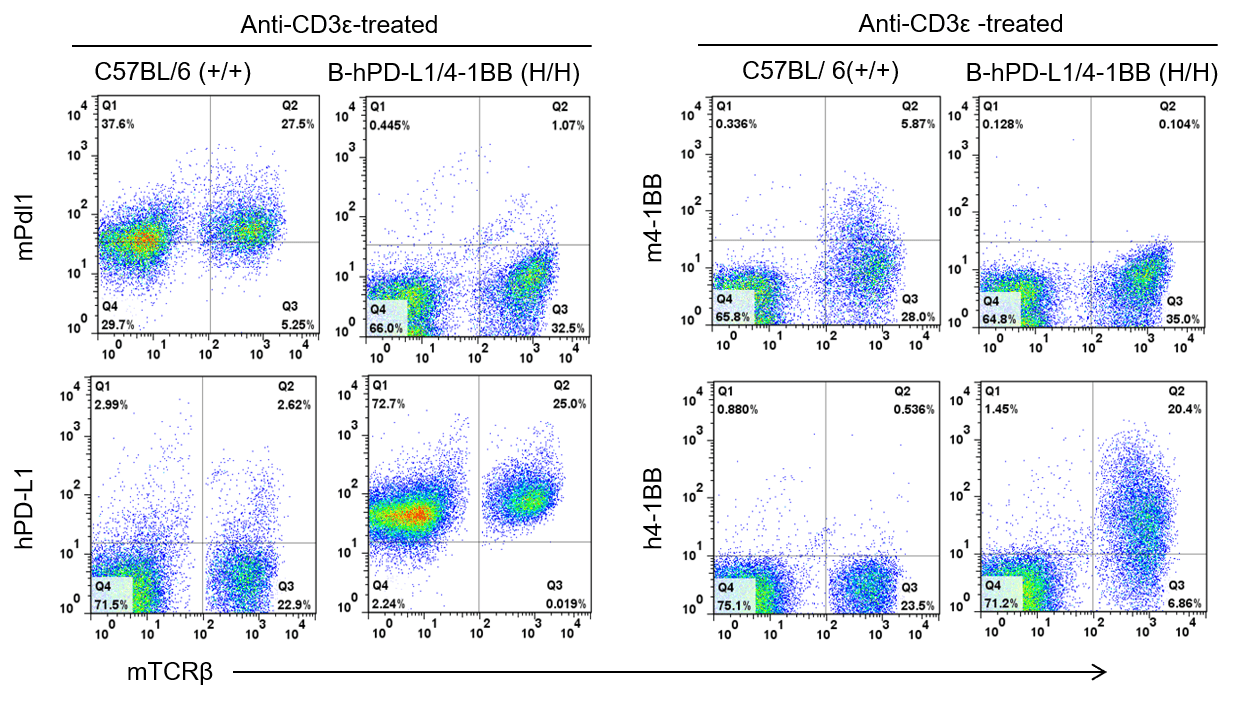
Strain specific PD-L1 and 4-1BB expression analysis in homozygous B-hPD-L1/h4-1BB mice by flow cytometry. Splenocytes were collected from WT and homozygous B-hPD-L1/h4-1BB (H/H) mice stimulated with anti-CD3ε in vivo, and analyzed by flow cytometry with species-specific anti-hPD-L1 and anti-h4-1BB antibody. Mouse Pdl1 and 4-1BB was detectable in WT mice but not in homozygous B-hPD-L1/h4-1BB mice. Human PD-L1 and 4-1BB was exclusively detectable in homozygous B-hPD-L1/h4-1BB mice but not in WT mice.
Application
In vivo efficacy of combination of anti-human PD-L1 antibody and anti-human 4-1BB antibody
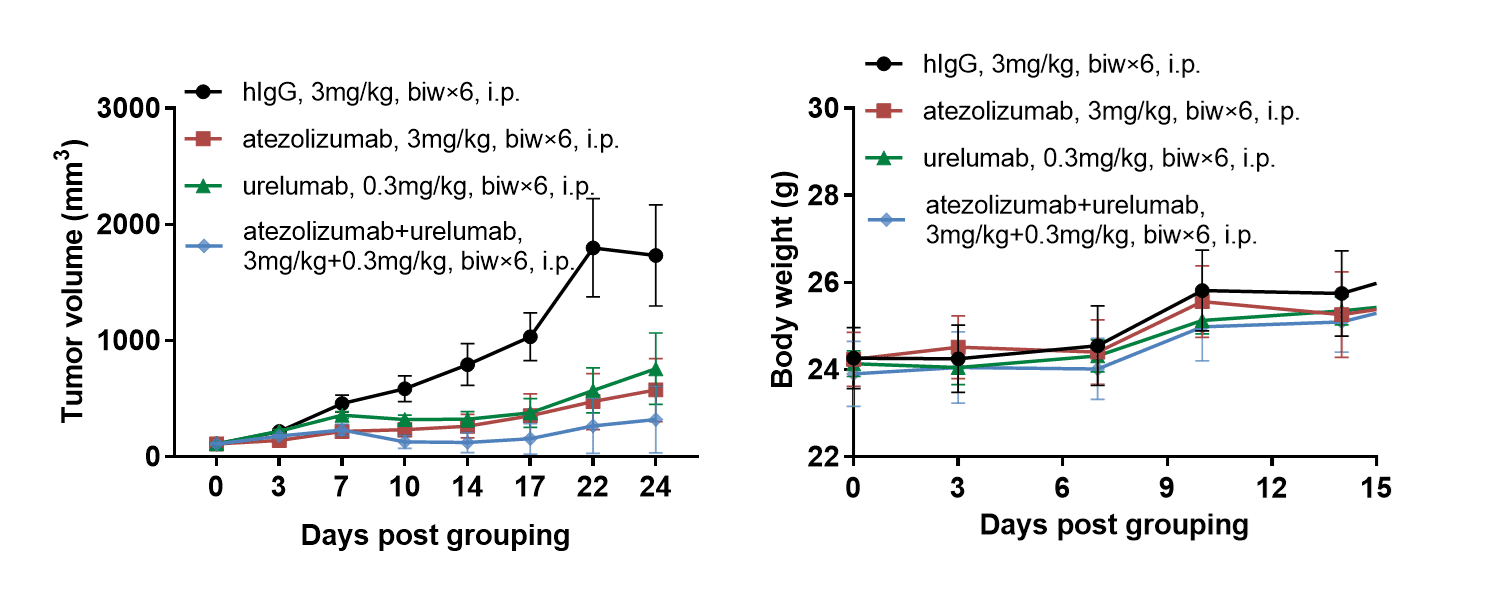
Antitumor activity of combination of anti-human PD-L1 antibody (atezolizumab,in house)and anti-human 4-1BB (urelumab, in house) in B-hPD-L1/h4-1BB mice. (A) combination of anti-human PD-L1 antibody and anti-human 4-1BB antibody inhibited MC38 tumor growth in B-hPD-L1/h4-1BB mice. Murine colon cancer MC38 cells were subcutaneously implanted into homozygous B-hPD-L1/h4-1BB mice (female, 6-week-old, n=6). Mice were grouped when tumor volume reached approximately 100 mm3, at which time they were treated with anti-human PD-L1 antibody and anti-human 4-1BB antibody with doses and schedules indicated in panel. (B) Body weight changes during treatment. As shown in panel A, combination of anti-human PD-L1 antibody and anti-human 4-1BB antibody were efficacious in controlling tumor growth in B-hPD-L1/h4-1BB mice, demonstrating that the B-hPD-L1/h4-1BB mice provide a powerful preclinical model for in vivo evaluation of combination of anti-human PD-L1 antibody and anti-human 4-1BB antibody. Values are expressed as mean ± SEM.
4-1BB-PD-L1 bispecific antibody Efficacy Evaluation
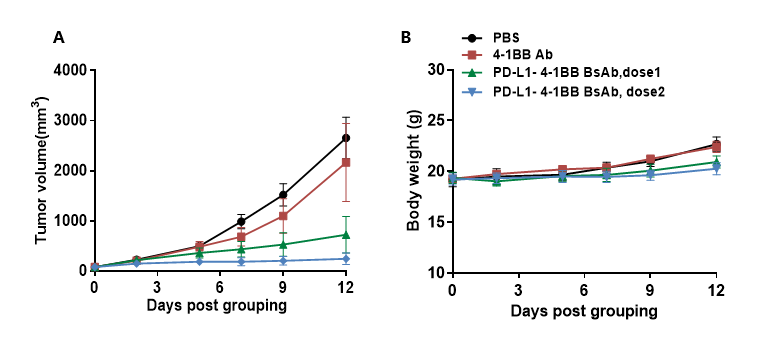
Antitumor activity of anti-4-1BB antibody and anti-4-1BB-PD-L1 bispecific antibody in B-hPD-L1/h4-1BB mice. (A) Anti-4-1BB antibody and anti-4-1BB-PD-L1 bispecific antibody inhibited B16F10-hPD-L1 tumor growth in B-hPD-L1/h4-1BB mice. B16F10-hPD-L1 cells were subcutaneously implanted into homozygous B-hPD-L1/h4-1BB mice (female, 7-week-old, n=6). Mice were grouped when tumor volume reached approximately 100 mm3, at which time they were treated with anti-4-1BB antibody and bispecific antibody with doses and schedules indicated in panel. (B) Body weight changes during treatment. As shown in panel A, the bispecific antibody were more efficacious in controlling tumor growth in B-hPD-L1/h4-1BB mice compared with PBS and the anti-4-1BB antibody group, demonstrating that the B-hPD-L1/h4-1BB mice provide a powerful preclinical model for in vivo evaluation of anti-human PD-L1 antibody, anti-human 4-1BB antibody or related bispecific antibody. Values are expressed as mean ± SEM.
-
Publication

-
- Jeong S, Park E, Kim HD, Sung E, Kim H, Jeon J, Kim Y, Jung UJ, Son YG, Hong Y, Lee H, Lee S, Lim Y, Won J, Jeon M, Hwang S, Fang L, Jiang W, Wang Z, Shin EC, Park SH, Jung J. Novel anti-4-1BB×PD-L1 bispecific antibody augments anti-tumor immunity through tumor-directed T-cell activation and checkpoint blockade. J Immunother Cancer. 2021 Jul;9(7):e002428. doi: 10.1136/jitc-2021-002428. PMID: 34230109.
- Zhai T, Wang C, Xu Y, Huang W, Yuan Z, Wang T, Dai S, Peng S, Pang T, Jiang W, Huang Y, Zou Y, Xu Y, Sun J, Gong X, Zhang J, Tsun A, Li B, Miao X. Generation of a safe and efficacious llama single-domain antibody fragment (vHH) targeting the membrane-proximal region of 4-1BB for engineering therapeutic bispecific antibodies for cancer. J Immunother Cancer. 2021 Jun;9(6):e002131. doi: 10.1136/jitc-2020-002131. PMID: 34172514; PMCID: PMC8237747.
-
References

-
- Trends Mol Med.2015 Jan;21(1):24-33. doi: 10.1016/j.molmed.2014.10.009. Epub 2014 Oct 30.
- Proc Natl Acad Sci U S A.2008 Feb 26;105(8):3011-6. doi: 10.1073/pnas.0712278105. Epub 2008 Feb 14.
- PLoS One.2014 Jan 21;9(1):e86337. doi: 10.1371/journal.pone.0086337. eCollection 2014
- Cancer Gene Ther. 2004 Mar;11(3):215-26.


