In Vivo Toxicity Testing & Efficacy Case Studies
In addition to our expertise in evaluating efficacy of novel antibody drugs, Biocytogen is known for its high quality in vivo toxicity studies. We offer toxicity analyses as both independent offerings and in support of in vivo efficacy studies, which include:
- Animal clinical observations
- Complete blood count (CBC)
- Blood chemistry analysis
- Histopathology (FFPE block preparation, H&E, IHC)
- On-mechanism toxicity assays
-
Case 1: Evaluating Anti-human CD40 In Vivo Efficacy And Safety in B-hCD40 Mice
-
1. Efficacy of anti-human CD40 antibody in B-hCD40 mice
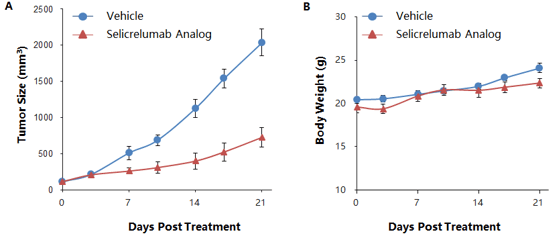
Murine colon cancer MC38 cells were subcutaneously implanted in homozygous B-hCD40 mice, where the extracellular domain of human CD40 replaces that of mouse CD40 via genomic knock-in. Mice were grouped when the average tumor size reached approximately 150±50 mm3 (n=5). Selicrelumab significantly inhibited tumor growth, confirming that the B-hCD40 mouse model is a powerful tool for in vivo studies of anti-human CD40 antibodies in mice. Tumor volume: mean ± SEM (A). Body weight: mean ± SEM (B)
2. Evaluation of anti-human CD40 toxicity in B-hCD40 mice by comprehensive analysis of CBC, blood chemistry, cytokine profiling, and immune cell profiling (flow cytometry)
A. Anti-human CD40 administration schedule and body weight over the course of treatment
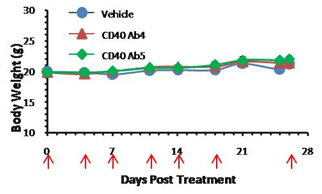
Anti-human CD40 antibodies were administered i.p. in homozygous B-hCD40 mice on days indicated by red arrows (n=5). No significant difference in average body weight was detected over the course of the treatment.
B. Complete blood count (CBC) of anti-human CD40-treated B-hCD40 mice
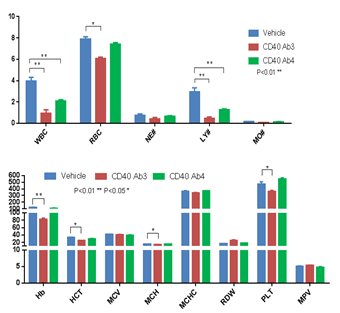
Abbreviation Meaning Abbreviation Meaning WBC White Blood Cell Count MCV Mean Corpuscular Volume NE# Neutrophil Count MCH Mean Corpuscular Hemoglobin LY# Lymphocyte Count MCHC Mean Corpuscular Hemoglobin Concentration MO# Monocyte Count MPV Mean Platelet Volume RBC Red Blood Cell Count PLT Platelet Count Hb Hemoglobin RDW Red Cell Distribution Width HCT Hematocrit CBC was performed with anti-CD40 antibodies in B-hCD40 mice two days after the termination of efficacy evaluation experiment.
C. Blood chemistry of anti-human CD40-treated B-hCD40 mice
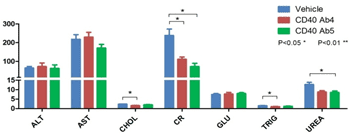
Abbreviation Meaning Explanation ALT Alanine transaminase High level indicates liver injured AST Aspartate transaminaseHigh level indicates liver damage CHOL Cholesterol High level indicates high blood liquid CR Creatinine High level indicates low glomerular filtration GLU Glucose Hyperglycemia or hypoglycemia TRIG Triacylglycerol High level indicates high blood lipid UREA Urea High level indicates kidney damage, liver disease, diabetes or infection The blood chemistry was tested with anti-human CD40 antibodies in B-hCD40 mice two days after the termination of efficacy evaluation experiment.
D. Cytokine analysis of anti-human CD40 antibody-treated B-hCTLA-4 mice
Cytokine profiles in anti-human CD40 antibody treated MC38-bearing B-hCD40 mice. Cytokines were measured by LEGENDplexTM (Biolegend). Anti-human CD40 Ab4 and Ab5 showed different cytokine profiles. Anti-human CD40 Ab4 treatment led to significant increase several cytokines including IFN-γ, MCP-1, and IL-27. Anti-human CD40 Ab5 resulted in significant increase of IL-10. Both antibodies modestly elevated TNF-α.
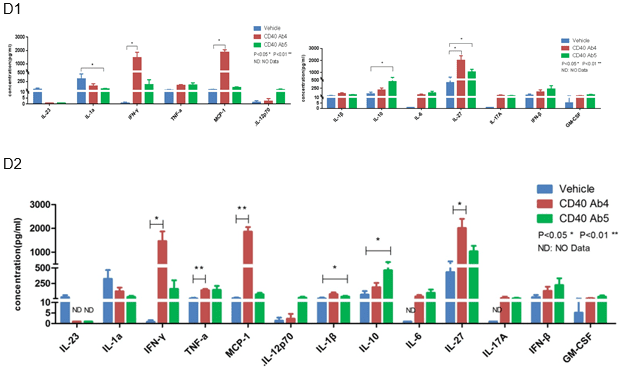
E. Immune cell profiling of anti-human CD40-treated B-hCD40 mice
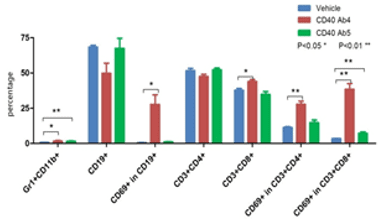
Flow cytometry was performed to examine immune cell profiles after anti-human CD40 antibody treatment in B-hCD40 mice. The results indicated that CD40 Ab4 treatment resulted in a significant increase in the percentage of activated CD4 and CD8 T cells and B cells.
F. Activation of B lymphocytes in B-hCD40 mice upon anti-human CD40 antibody treatment
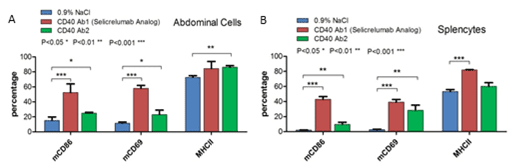
Anti-human CD40 antibodies were administered i.p. in homozygous B-hCD40 mice After 48h, abdominal cells and splenocytes were collected and analyzed for expression of activation markers by flow cytometry. Selcrelumab led to significant elevation of activation markers in both spleen and abdomen.
3. Evaluation of anti-human CD40 toxicity in B-hPD-1/hCD40 mice by CBC, blood chemistry, and histopathology
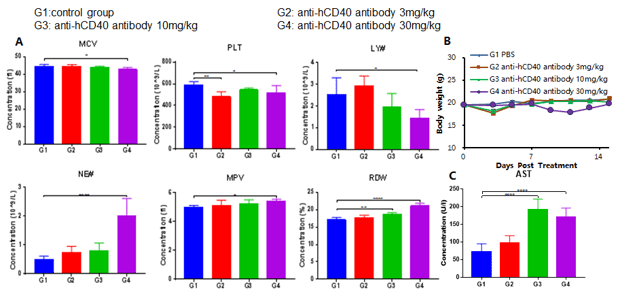
Abbreviation Meaning Abbreviation Meaning WBC White Blood Cell Count MCV Mean Corpuscular Volume NE# Neutrophil Count MCH Mean Corpuscular Hemoglobin LY# Lymphocyte Count MCHC Mean Corpuscular Hemoglobin Concentration MO# Monocyte Count MPV Mean Platelet Volume RBC Red Blood Cell Count PLT Platelet Count Hb Hemoglobin RDW Red Cell Distribution Width HCT Hematocrit 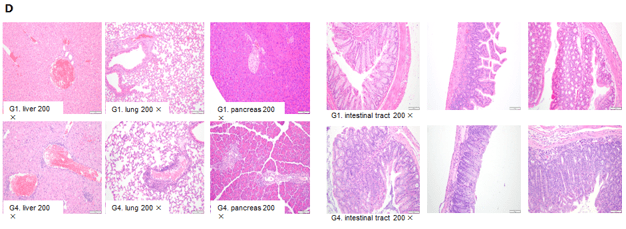
- Effect of an anti-human CD40 antibody in CBC test in B-hPD-L1/hCD40 mice, where the extracellular domains of both hPD-L1 and hCD40 replace their respective murine counterpart via genomic knock-in. Antibody treatment 6 days after the initial dose led to decrease of MCV, PLT and LY# and increase of NE#, MPV and RDW.
- Body weight profile over the course of after antibody administration. No persistent body weight changes were observed.
- AST of B-hPD-1/hCD40 mice treated with higher doses increased significantly compared to vehicle control group. Microscopic changes were observed in B-hPD-1/hCD40 mice from the anti-hCD40 antibody treated group of 30mg/kg.
- Elevated infiltration of mixed inflammation cells in the interstitium as shown by H&E staining of liver, lung, pancreas, and intestine.
-
Case 2: Evaluating In Vivo Efficacy And Safety of Anti-human CD47 Antibody in B-hCD47/hSIRPA Mice
-
1. Efficacy and toxicity of anti-human CD47 antibody in B-hCD47/hSIRPA mice
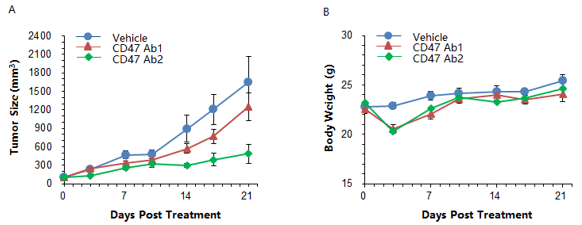
Colon cancer MC38-hCD47 cells engineered to express human CD47 were subcutaneously implanted in homozygous B-hCD47/hSIRPA mice, where the extracellular domains of both hCD47 and hCSIPRA replace their respective murine counterpart via genomic knock-in. The mice were grouped as the average tumor volume reached 150±50mm3 (n=5). Anti-human CD47 antibodies demonstrated anti-tumor efficacy in this model. Antibody treatment also resulted in a rapid but moderate body weight loss, which was recovered by day 10. These results indicate that B-hCD47/hSIRPA mice are a useful model for testing anti-human hCD47 antibody efficacy and observing whole body toxicity. Data is expressed as mean ± SEM of tumor volume and body weight.
2. Evaluating hematological and liver toxicity of anti-human CD47 antibodies in B-hCD47/hSIRPA mice
A. Indication of hematological toxicity by anti-human CD47 antibodies
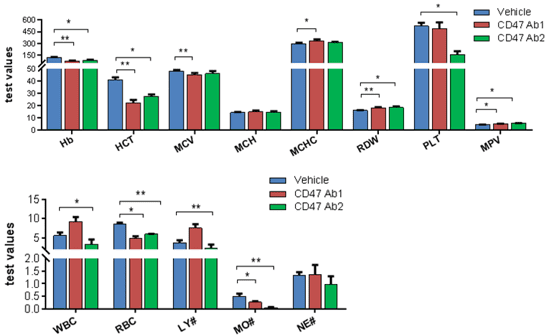
Indication of hematological toxicity of Anti-hCD47 antibodies administered i.p. in homozygous B-hCD47/hSIRPA mice. Blood were collected two days after the treatment. Significant decrease of red blood cell counts (RBC), hematocrit (HCT), were observed upon anti-human CD47 treatment. Monocyte counts were decreased. Anti-human CD47 Ab2 also led to decrease of platelet count.
Abbreviation Meaning Abbreviation Meaning WBC White Blood Cell Count MCV Mean Corpuscular Volume NE# Neutrophil Count MCH Mean Corpuscular Hemoglobin LY# Lymphocyte Count MCHC Mean Corpuscular Hemoglobin Concentration MO# Monocyte Count MPV Mean Platelet Volume RBC Red Blood Cell Count PLT Platelet Count Hb Hemoglobin RDW Red Cell Distribution Width HCT Hematocrit Anti-hCD47 antibodies were i.p. injected into homozygous hSIRPA/hCD47 mice. Blood were collected two days after the treatment and analyzed by Blood Routine Test.
B. Liver toxicity of anti-human CD47 antibodies
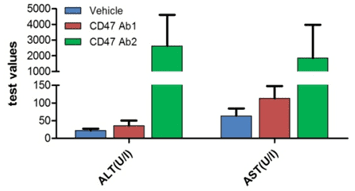
Abbreviation Meaning Explanation ALT Alanine transaminase Elevated level indicates liver damage AST Aspartate transaminase Elevated level indicates liver damage Anti-hCD47 antibodies led to liver damage as indicated by elevated levels of liver enzymes in blood in homozygous hSIRPA/hCD47 mice. Blood was collected two days after the initial treatment.



