Rheumatoid Arthritis Model Introduction
- Rheumatoid Arthritis (RA) is among the most common autoimmune diseases. Clinical symptoms of RA include generalized joint swelling, pain and motion disorder. Severe cases of RA lead to disability, as evidenced by hyperplastic synovitis, cartilage injury and bone structure destruction.
- Collagen-induced arthritis (CIA) is a common experimental rheumatoid arthritis mouse model induced by immunization with type II collagen (CII), the major constituent protein of articular cartilage. Animal models immunized with CII develop autoimmune polyarthritis, which shares several clinical and histological features with rheumatoid arthritis.
- Collagen antibody-induced arthritis (CAIA) is induced by directly injecting anti-collagen antibodies, resulting in a shorter time to disease onset.
At Biocytogen, we have established stable rheumatoid arthritis mouse models in different strains of mice (C57BL/6, DBA, BALB/c), which can be used for pharmacodynamic evaluation of RA-related drugs.
-
CIA Model Induction
-
Experimental mouse strains: C57BL/6, DBA1, 10-13 weeks old, female
Modeling reagent: CII emulsion
Modeling method: Sensitization, day 0; Challenge, day 21
-
Clinical Score and Pathological Analysis of C57BL/6 CIA Mice
-
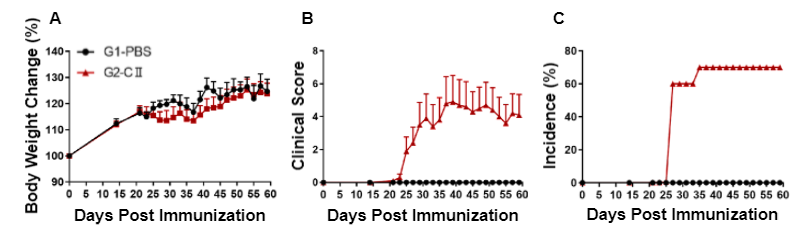
Arthritis was induced in wild-type C57BL/6 mice by type II collagen (CII) immunization. (A) Body weight change, (B) clinical score, and (C) incidence were measured in wild-type C57BL/6 mice exposed to PBS control (G1) or CII (G2).
Clinical score (generalized joint swelling, pain and impaired motion) was significantly increased in wild-type C57BL/6 mice treated with CII compared to vehicle control, indicative of an established rheumatoid arthritis mouse model.
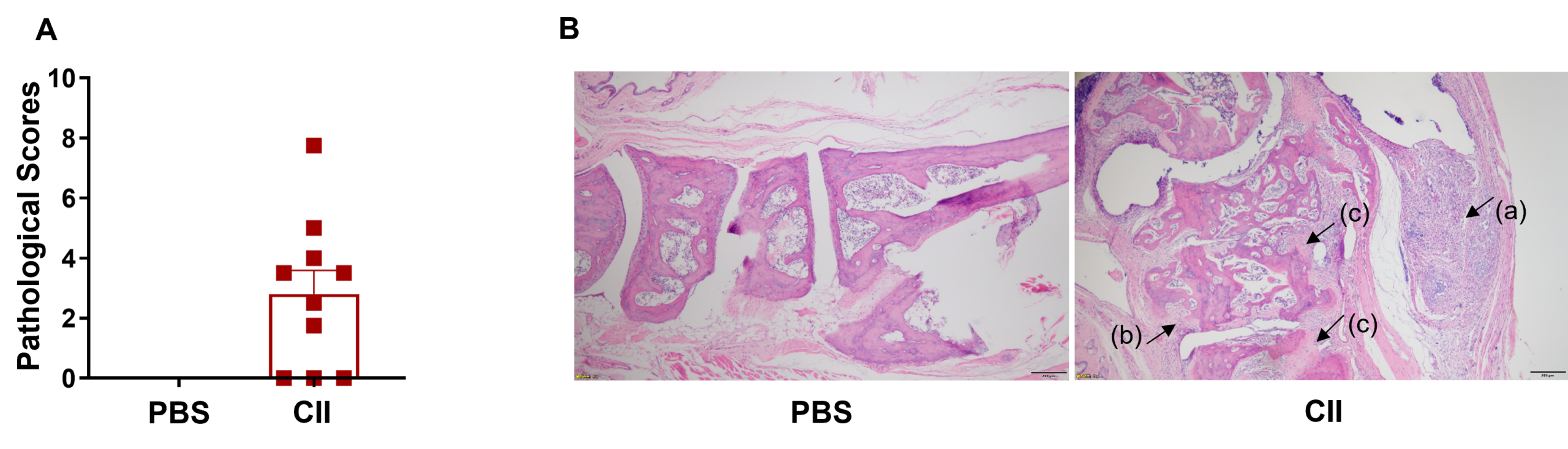
Pathological analysis. (A) Pathological scores, and (B) hematoxylin and eosin (H&E) staining of joints in wild-type C57BL/6 mice treated with PBS (G1) or CII (G2).
Joints in the PBS-treated mice (G1) showed normal articular structure with null disease scores. Joints in the mice (G2) treated with CII displayed (a) mixed inflammatory cell infiltration, (b) periarticular stenosis, (c) articular cartilage and bone tissue destruction, as well as other arthritic lesions in all or parts of the limb joints. These data suggest that the rheumatoid arthritis mouse model, via CII emulsion, was successfully established.
-
Methotrexate Efficacy Validation in C57BL/6 CIA Mice
-
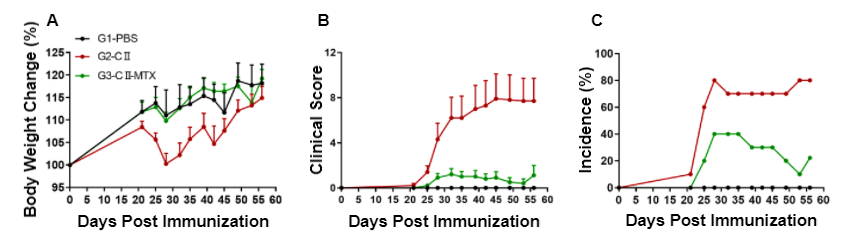
Pharmacodynamic effects of methotrexate (MTX) in wild-type C57BL/6-arthritic mice. (A) Body weight change, (B) clinical score, and (C) incidence of morbidity were measured in wild-type C57BL/6 mice exposed to PBS control (G1), CII (G3), or CII in combination with methotrexate (G3).
Wild-type C57BL/6 mice exposed to CII (G2) exhibited significantly higher clinical scores, in addition to more obvious body weight fluctuation compared to wild-type C57BL/6 control mice (G1). Following administration of methotrexate in combination with CII (G3), the mean clinical score was significantly lower than that of the CII group alone. Additionally, the maximum incidence of the CII-methotrexate group was significantly lower at 40% compared to the 80% incidence rate of the CII alone group. Altogether, this data suggests methotrexate effectively alleviates disease related symptoms in a rheumatoid arthritis mouse model.
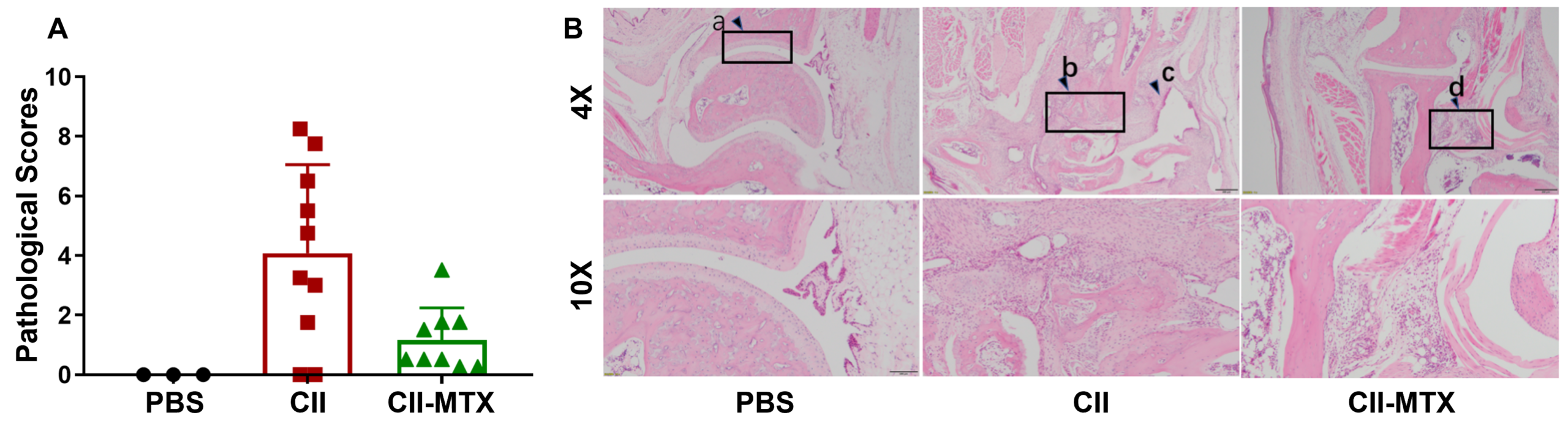
Pathological analysis of wild-type C57BL/6-arthritic mice treated with methotrexate. (A) Pathological scores, and (B) hematoxylin and eosin (H&E) staining of joints in wild-type C57BL/6 mice exposed to PBS control (G1), CII (G3), or CII in combination with methotrexate (G3).
Compared to control wild-type C57BL/6 mice that showed smooth ankle cartilage (a), mice treated with CII alone displayed subcutaneous mixed inflammatory cell infiltration, synovitis and/or pannus formation (c), articular cartilage destruction, disappearance of joint cavity and partial bone tissue fusion (b) were observed in ankle joint tissues. Despite edema and inflammatory cell infiltration (d) in the subcutaneous ankle tissue of CII mice treated with methotrexate, the mean pathological score was significantly lower than the CII alone group, further demonstrating that methotrexate provides a therapeutic effect on arthritic lesions in CIA mice.
-
Human IL6 and IL6R Expression in Humanized B-hIL6/hIL6R Mice
-
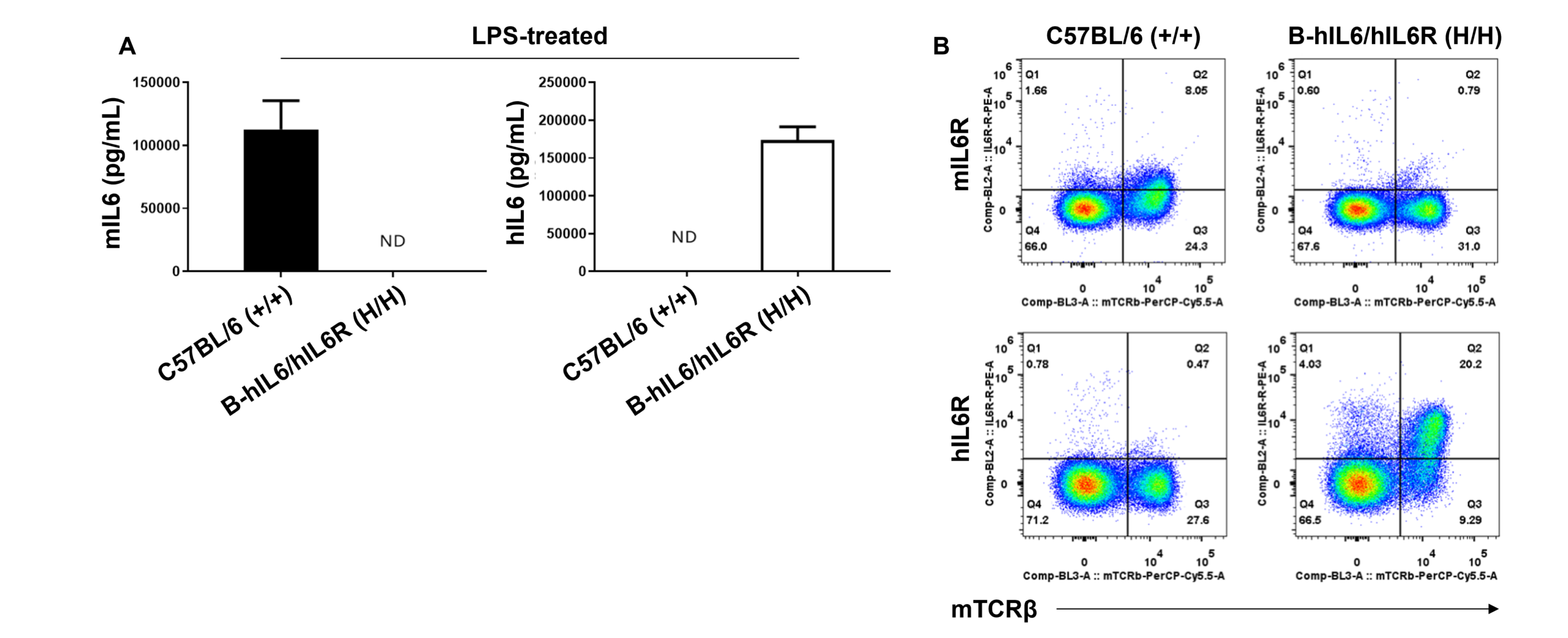
Species-specific IL6 and IL6R expression in homozygous humanized B-hIL6/hIL6R mice. (A) Following LPS stimulation in vivo, serum was collected from wild-type C57BL/6 (+/+) and homozygous B-hIL6/hIL6R (H/H) mice and analyzed using a species-specific IL6 ELISA kit. (B) Splenocytes were isolated from wild-type C57BL/6 (+/+) and homozygous B-hIL6/hIL6R (H/H) mice and analyzed by flow cytometry using a species-specific anti-IL6R antibody.
These results suggest human IL6 and IL6R is exclusively expressed in humanized B-hIL6/hIL6R mice compared to wild-type C57BL/6 mice.
-
Sirukumab Efficacy Validation in Humanized B-hIL6/hIL6R CIA Mouse Models
-
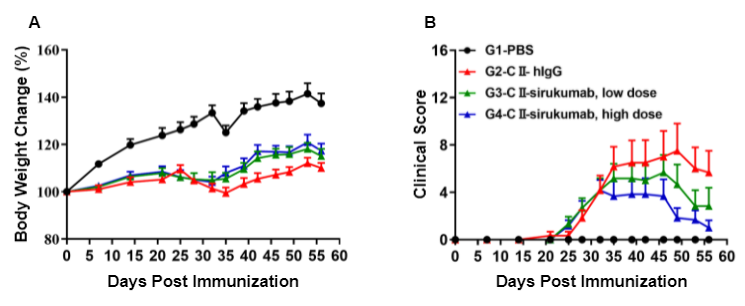
Pharmacodynamic effects of an anti-human IL6 antibody in homozyogous humanized B-hIL6/hIL6R-arthritic mice. (A) Body weight change, and (B) clinical score were measured in humanized B-hIL6/hIL6R mice exposed to PBS control (G1), CII (G2), or CII in combination with different doses of sirukumab (G3, G4).
While clinical score was significantly increased in humanized B-hIL6/hIL6R mice treated with CII compared to vehicle control, administration of sirukumab showed improved clinical scores in a dose-dependent manner.
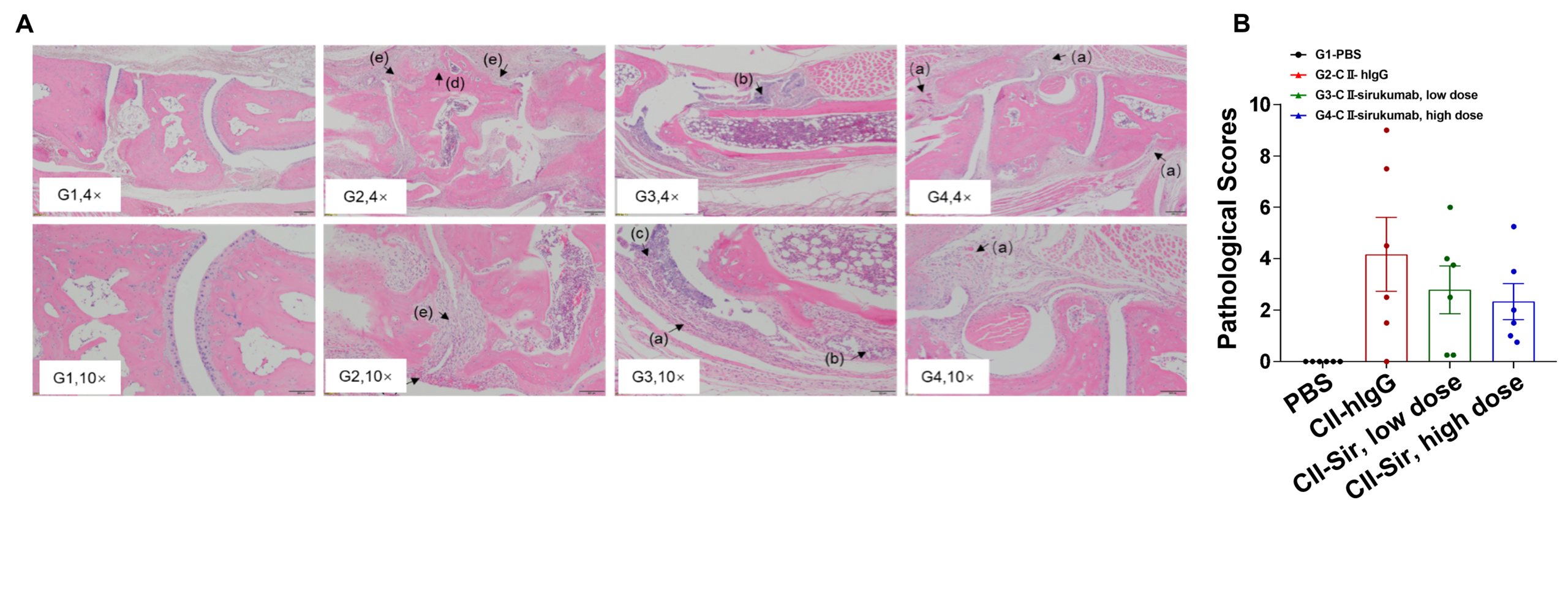 Efficacy of anti-human IL6 antibody in homozygous humanized B-hIL6/hIL6R-arthritic mice. (A) Hematoxylin and eosin (H&E) staining and (B) arthritis histology score on the joints of the extremities were observed and measured in humanized B-hIL6/hIL6R mice exposed to PBS control (G1), CII (G2), or CII in combination with different doses of sirukumab (G3, G4).
Efficacy of anti-human IL6 antibody in homozygous humanized B-hIL6/hIL6R-arthritic mice. (A) Hematoxylin and eosin (H&E) staining and (B) arthritis histology score on the joints of the extremities were observed and measured in humanized B-hIL6/hIL6R mice exposed to PBS control (G1), CII (G2), or CII in combination with different doses of sirukumab (G3, G4). Humanized B-hIL6/hIL6R mice treated with CII displayed bone structure damage (d), articular cavity or disappearance of periarticular space (e) and pannus (a) compared to vehicle control. Low doses of sirukumab showed slight inflammatory cell infiltration (b), pannus (a) and synovial hyperplasia (c), while high doses of sirukumab exhibited greatest therapeutic effects with partial pannus and reduced disease scores.
-
CAIA (Collagen Antibody-Induced Arthritis) Mouse Model Induction
-
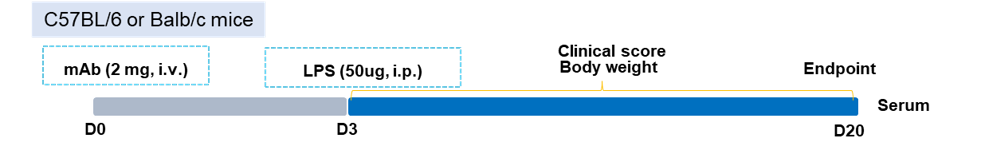
Experimental mouse strains: C57BL/6, BALB/c
Modeling reagent: CII mAb cocktail
Modeling method: Antibody cocktail, day 0; LPS, day 3
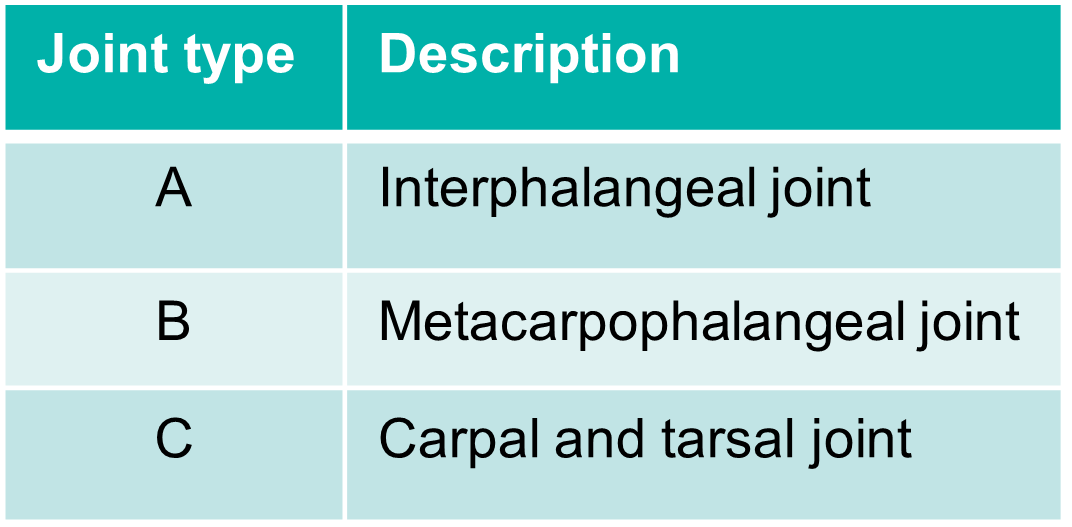
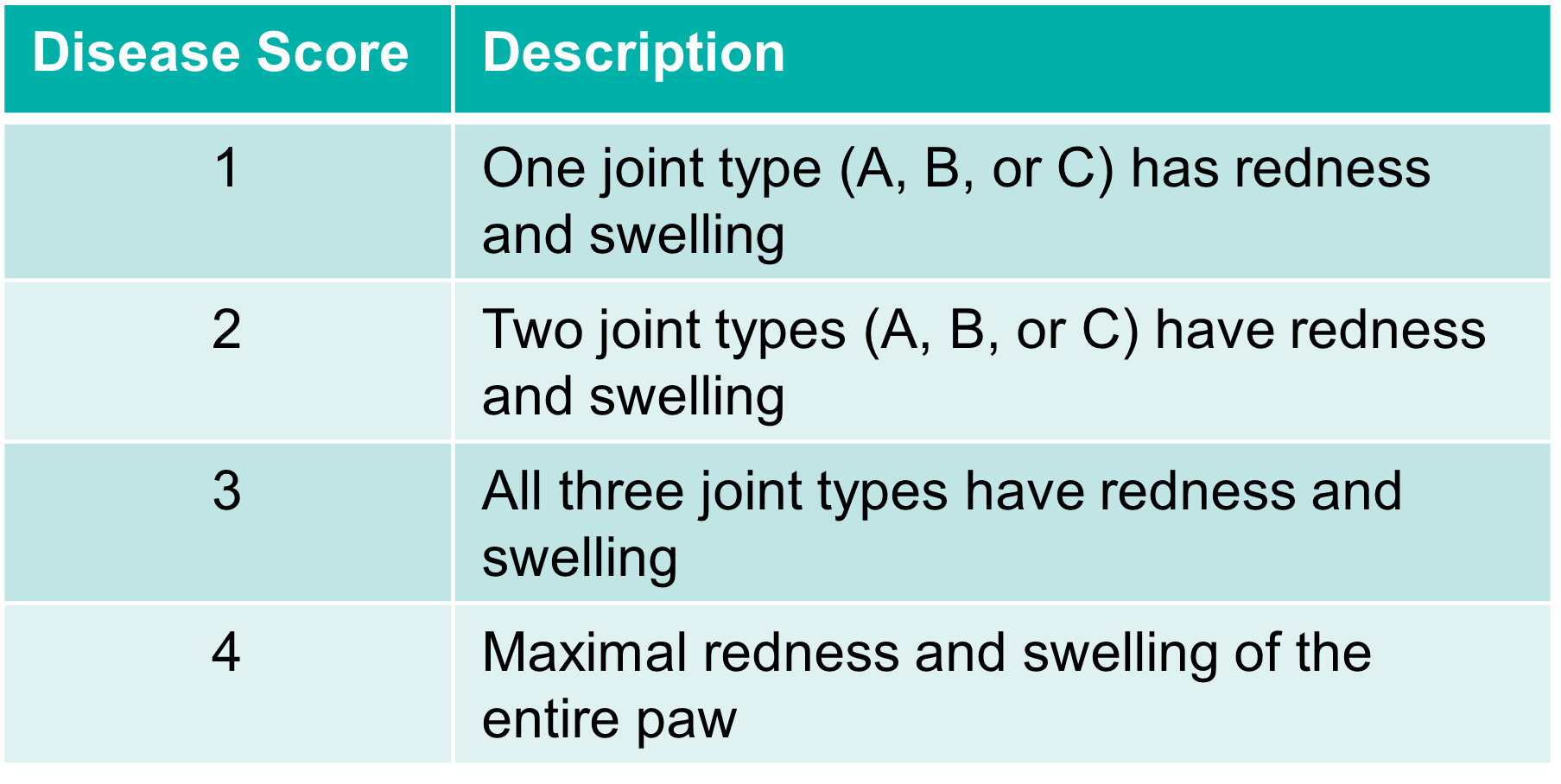
Scoring method:
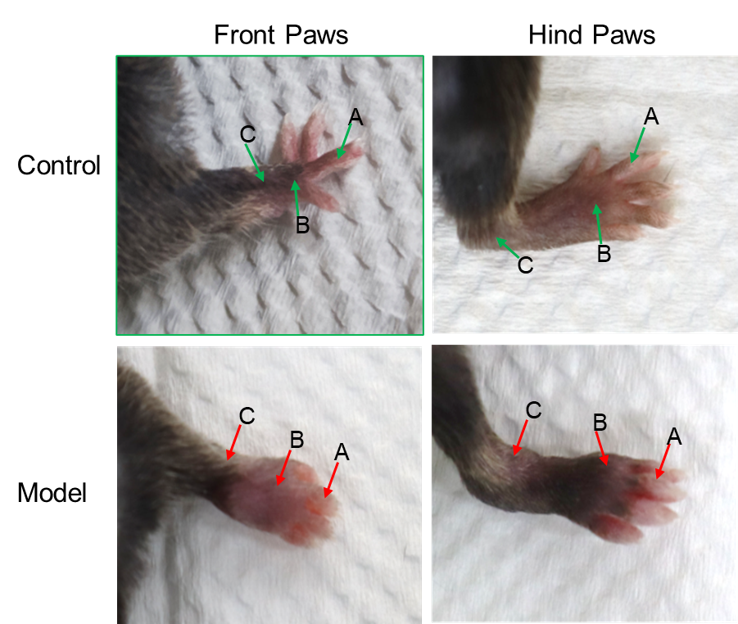
Swelling of the joints in the CAIA mouse model.
-
CAIA (Collagen Antibody-Induced Arthritis) Pathogenesis
-
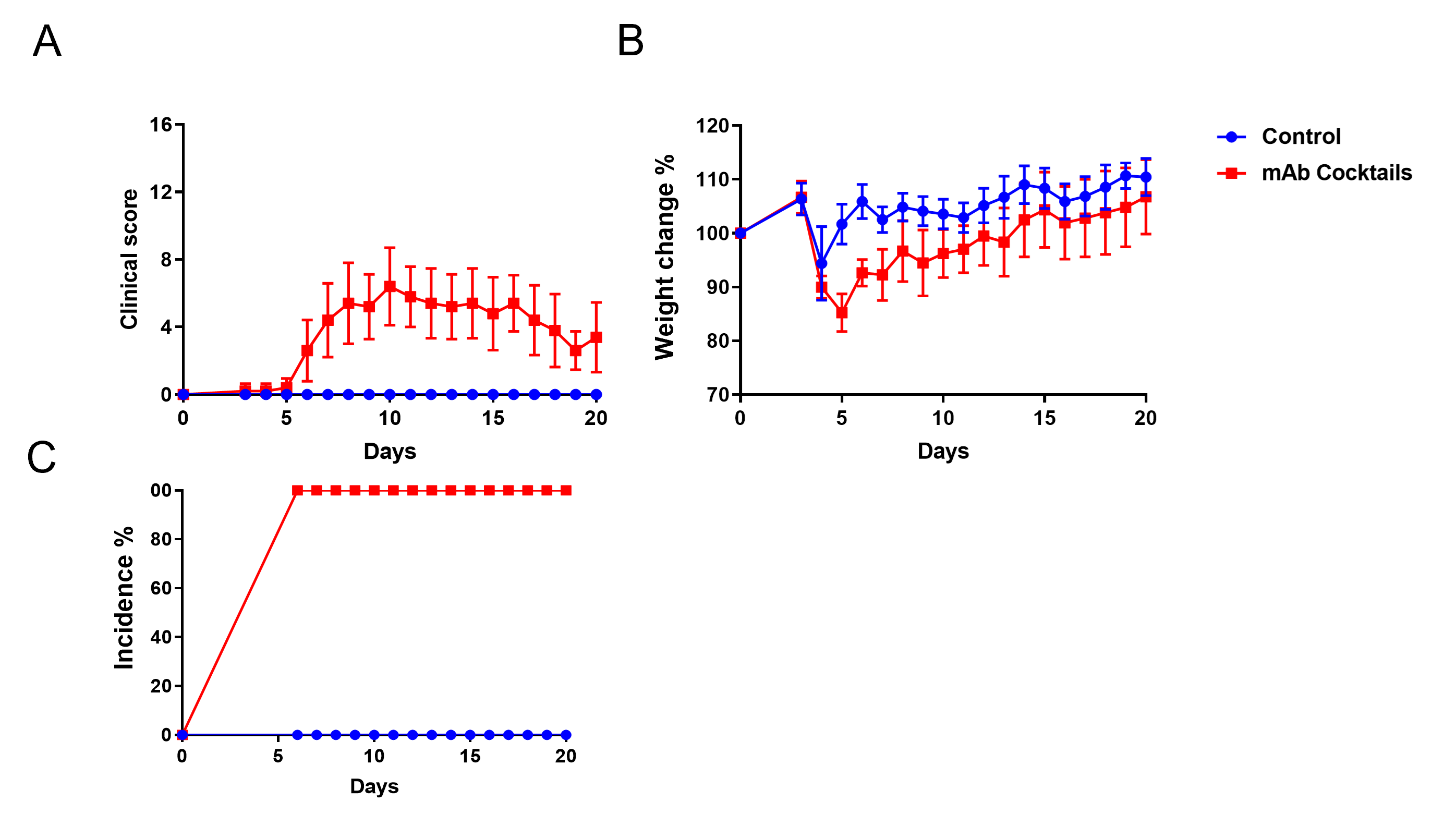
Quantitation of CAIA induction as measured by disease score and weight changes. Incidence of the disease is 100% in this example.
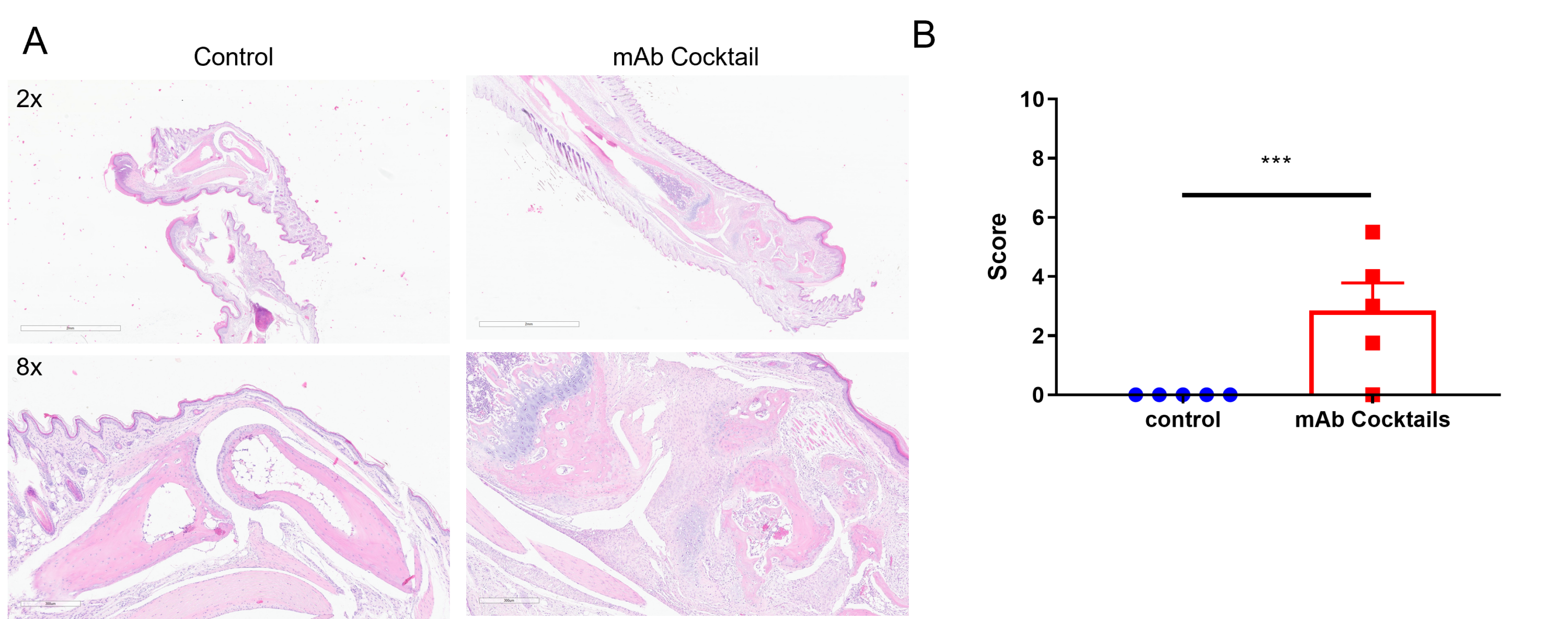
Tissue sections were stained with (H&E) (A), and infiltrating inflammatory cells (B) were scored. Together, these data indicate marked increases in swelling and inflammation in the CAIA model.



