Ex vivo PK/PD Assays & In vitro Pharmacology Services
Our pharmacology platform has extensive capabilities that extend beyond in vivo efficacy and toxicity studies. We have a dedicated team that provides support for ex vivo pharmacodynamic (PD) and pharmacokinetic (PK) assays, and in vitro primary and cultured cell-based assays.
Ex vivo pharmacology services include the following assays:
Pharmacodynamic (PD) services:
- Cytokine profiling services (Cytokine bead array, ELISA, Luminex, MSD, Elispot)
- Receptor occupancy (Flow-based)
- Immune cell profiling (Flow-based, H&E, IF, IHC)
- Target/biomarker expression (dPCR, IF, qPCR, RNA scope)
Pharmacokinetic (PK) services:
- Dose range finding/maximum tolerated dose studies (DRF/MTD)
- Drug administration by your preferred route:
- Intravenous (i.v.)
- Intraperitoneal (i.p.)
- Subcutaneous (s.c.)
- Per os (p.o.)
- Intrathecal (i.t.)
- Intramuscular (i.m.)
- Sample collection over specified time points from blood, bone marrow, tumor, spleen, liver and other tissues for biomarker or toxicity assessments (Histology, CBC, blood biochemistry, etc.)
- Blood and tissue-specific PK analysis for biotherapeutics where detection antibodies are available (ELISA, MSD)
- Bioavailability/biodistribution assessments, including tissue collection and concentration of biotherapeutics where detection antibodies are available (ELISA, MSD)
In vitro pharmacology services include the following assays:
- Antibody-dependent cellular cytotoxicity (ADCC) (LDH, Luminescence, Flow-based)
- Antibody-dependent cellular phagocytosis (ADCP) (Flow-based)
- Complement-dependent cytotoxicity (CDC) (CTG, Prestoblue)
- Drug blocking assays (Flow-based)
- Drug-cell binding assays (Flow-based)
- Endocytosis (Flow-based, IncuCyte)
- Hemolysis/coagulation tests (Luminex)
- In vitro stimulation assays (ELISPOT, Luminex)
- Mixed lymphocyte reaction (MLR) (ELISA, Flow-based, Luminex)
Some example studies are provided below. For more information about our pharmacology service capabilities, contact our experts today!
Watch the webinar:
Preclinical Pharmacokinetics (PK) and Pharmacodynamics (PD) Evaluation with Biocytogen
Example Studies
-
Cytokine Profiling (MSD)
-
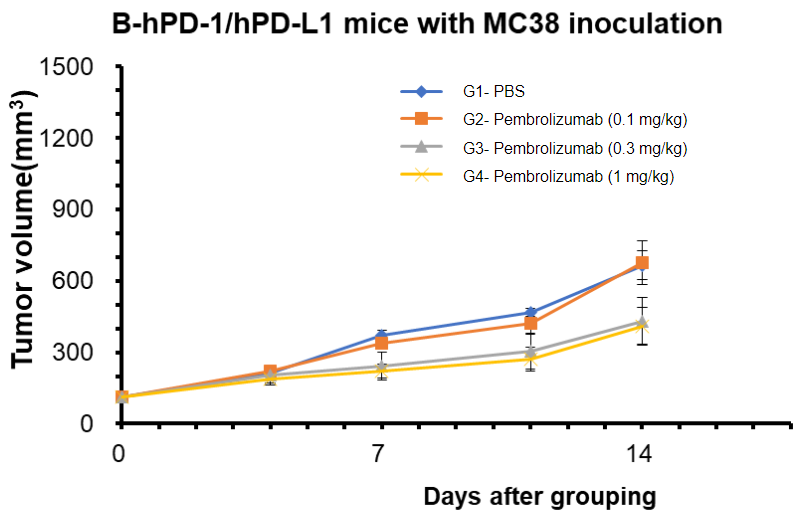
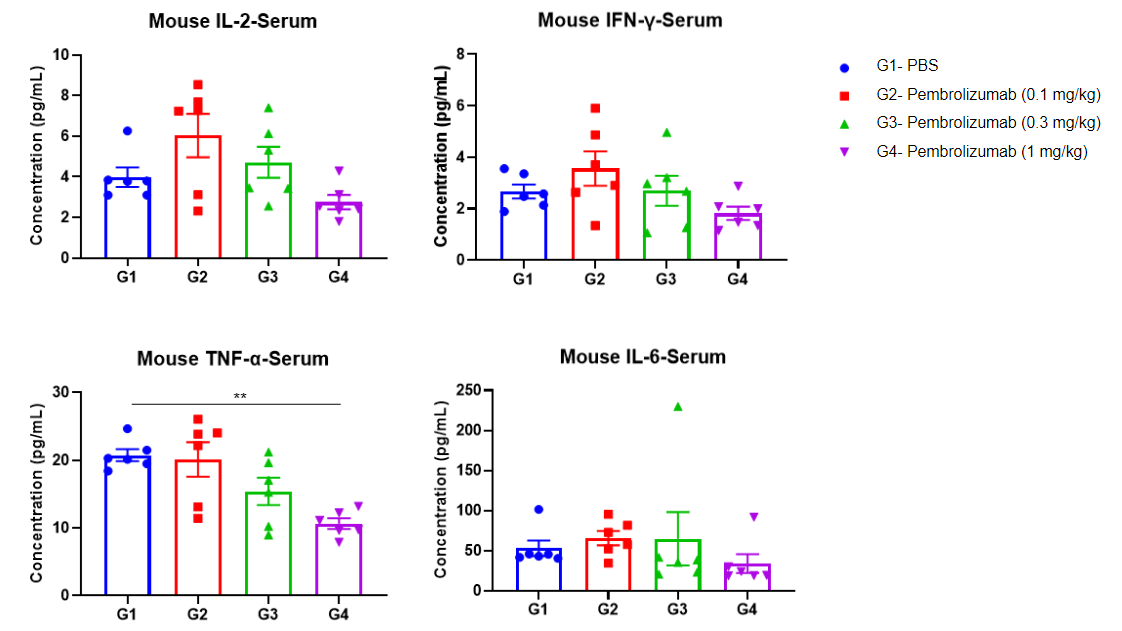
Serum cytokine profile of B-hPD-1/hPD-L1 mice harboring MC38 tumors treated with pembrolizumab. Samples were measured using MSD technology.
-
Cytokine Profiling (Luminex)
-
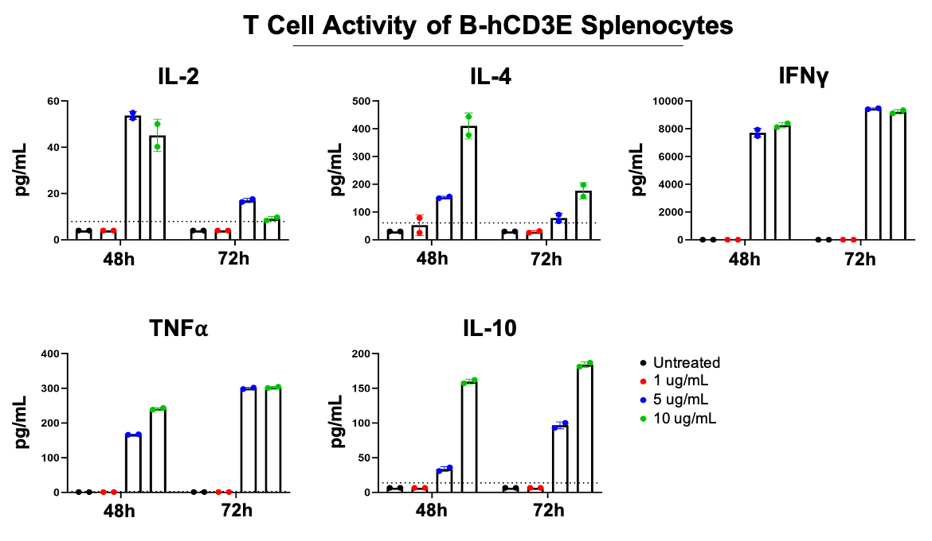
Splenocytes were isolated from humanized B-hCD3E mice and stimulated with varying concentrations of anti-human CD3 and anti-mouse CD28 antibodies for 72 hours. Cytokine secretion was measured by Luminex. These data indicate that humanized B-hCD3E mice can respond to human CD3, and illustrate the differential kinetics of the cytokine response.
-
Dose Range Finding
-
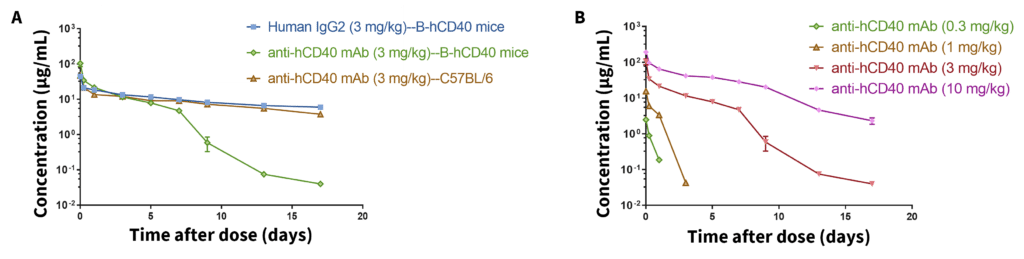
(A) Serum concentration of an anti-human CD40 antibody after intravenous (i.v.) administration in wild-type C57BL/6 and humanized B-hCD40 mice was measured. The anti-human CD40 antibody was cleared by target-mediated drug disposition (TMDD) in humanized B-hCD40 mice. (B) When higher doses of an anti-human CD40 antibody were used in B-hCD40 mice, the antibody remained in circulation for more than 2 weeks.
-
Tissue TA Distribution (IVIS)
-
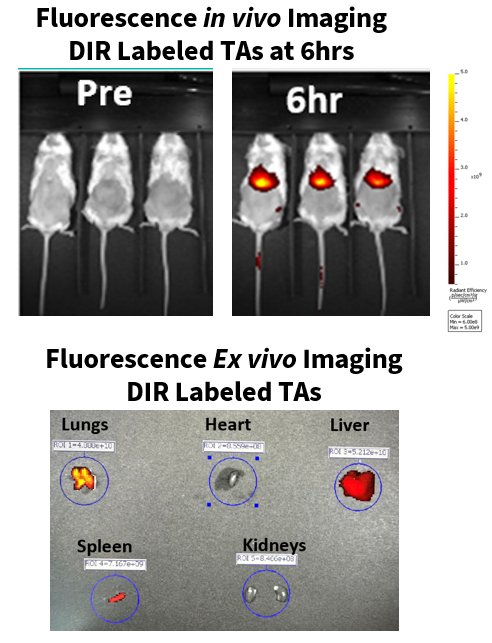
In this example, in vivo fluorescence imaging (IVIS Lumina S5) was employed to monitor and determine the distribution/accumulation of DIR-labeled compounds in B-NDG mice.
(Top) Post-intravenous administration, DIR-labeled TAs accumulated in the liver of B-NDG mice after 6 hours. (Bottom) Ex vivo fluorescence imaging of harvested tissues confirmed the accumulation of DIR-labeled test articles in the lungs, liver, and spleen, but not in the heart or kidneys.
-
Receptor Occupancy
-
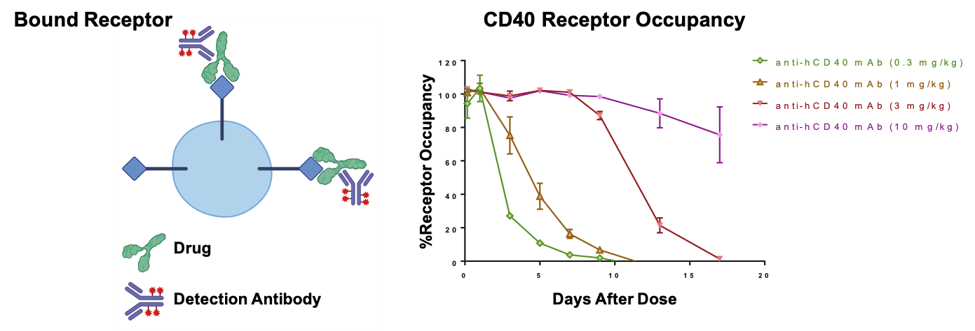
Humanized B-hCD40 mice were treated with different doses of an anti-human CD40 antibody, and the percentage of receptor occupancy on B cells isolated from peripheral blood mononuclear cells (PBMCs) was assessed by flow cytometry.
-
Pharmacokinetics Assessment
-
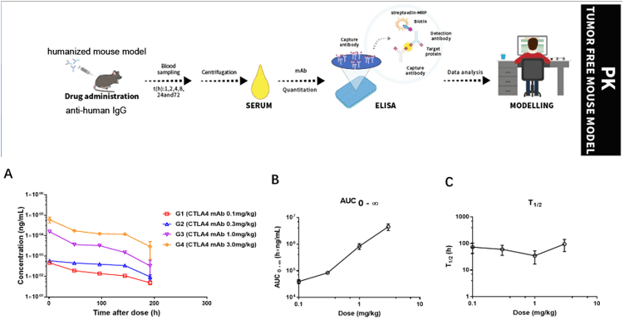
Pharmacokinetic (PK) analysis of an anti-human CTLA4 antibody in MC38 tumor-bearing humanized B-hCTLA4 mice, where the extracellular domain of human CTLA4 replaces that of mouse CTLA4 via genomic knock-in. Anti-human CTLA4 antibody was administered (i.v.) at varying concentrations. Plasma antibody concentrations were determined by ELISA (A) and PK parameters were derived by the non-compartmental model with Phoenix Winnolin 8.0. AUC0-∞ showed dose linearity (B) while half time (T1/2) (C) was unchanged across the doses administered.
-
Drug Toxicity Evaluation
-
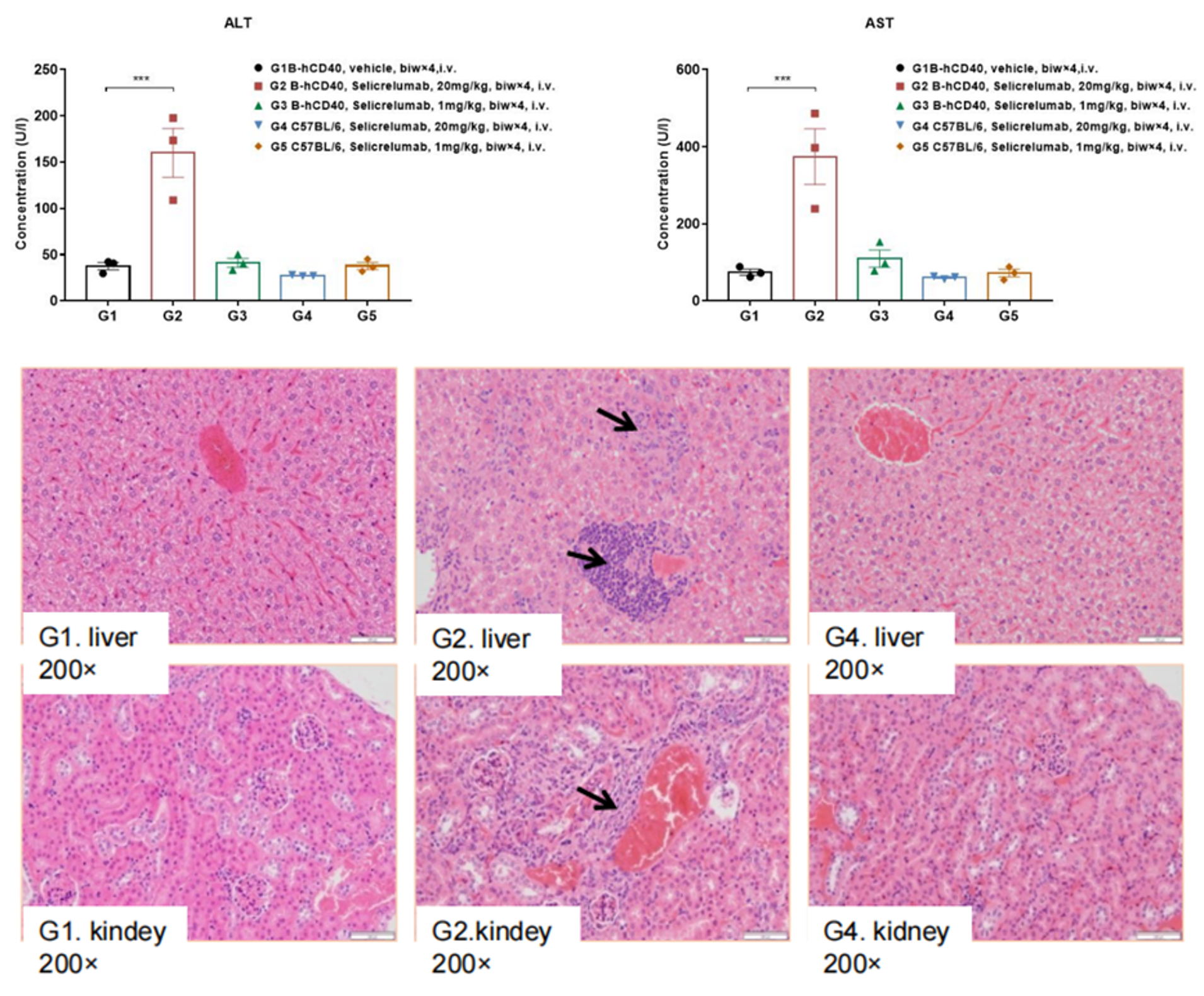
Humanized B-hCD40 mice were treated with Selicrelumab or vehicle (biw x 4, i.v.) for 2 weeks. AST and ALT were measured from the serum, and liver sections were stained with H&E for pathologic assessments. Selicrelumab treatment at 20mg/kg exhibited signs of liver stress and inflammatory infiltrate, indicating drug toxicity.
-
Mixed Lymphocyte Reaction (MLR)
-
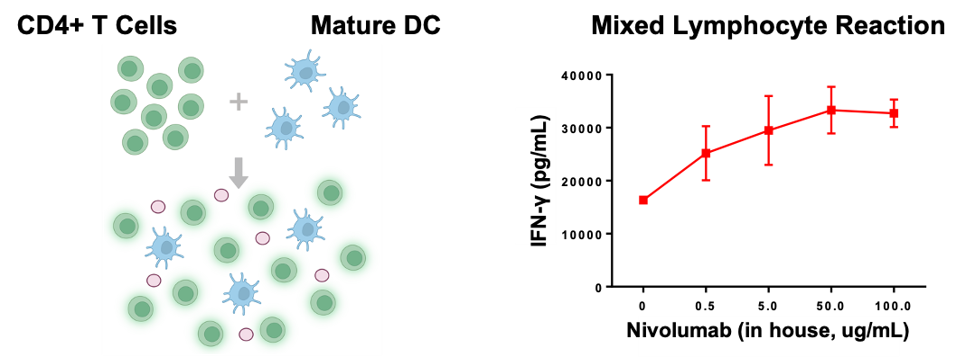
The mixed lymphocyte reaction (MLR) assay typically involves co-incubation of immune cell subtypes to trigger their activation. In this study, naïve CD4 T cells were incubated with mature dendritic cells and treated with a Nivolumab analog. After 120 hours, cytokine secretion was measured by ELISA.
-
Antibody-Dependent Cellular Cytotoxicity (Flow-based)
-
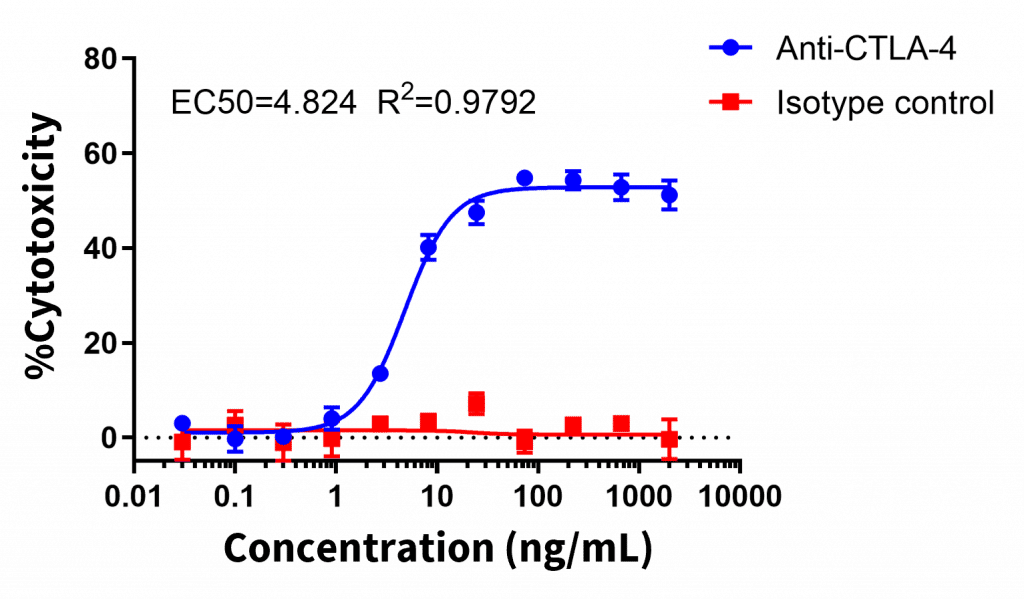
Jurkat cells expressing human CTLA-4 were mixed with peripheral blood mononuclear cells (PBMCs) and treated with a CTLA-4 antibody. Cytotoxicity was measured by flow cytometry using a viability dye. Alternative approaches to assessing antibody-dependent cellular cytotoxicity (ADCC) include evaluating CD107a upregulation by flow cytometry, LDH release, or using effector cells engineered to express luciferase in response to CD16A engagement (plate reader based-96 well format).
-
Complement-Dependent Cytotoxicity (Flow-based)
-
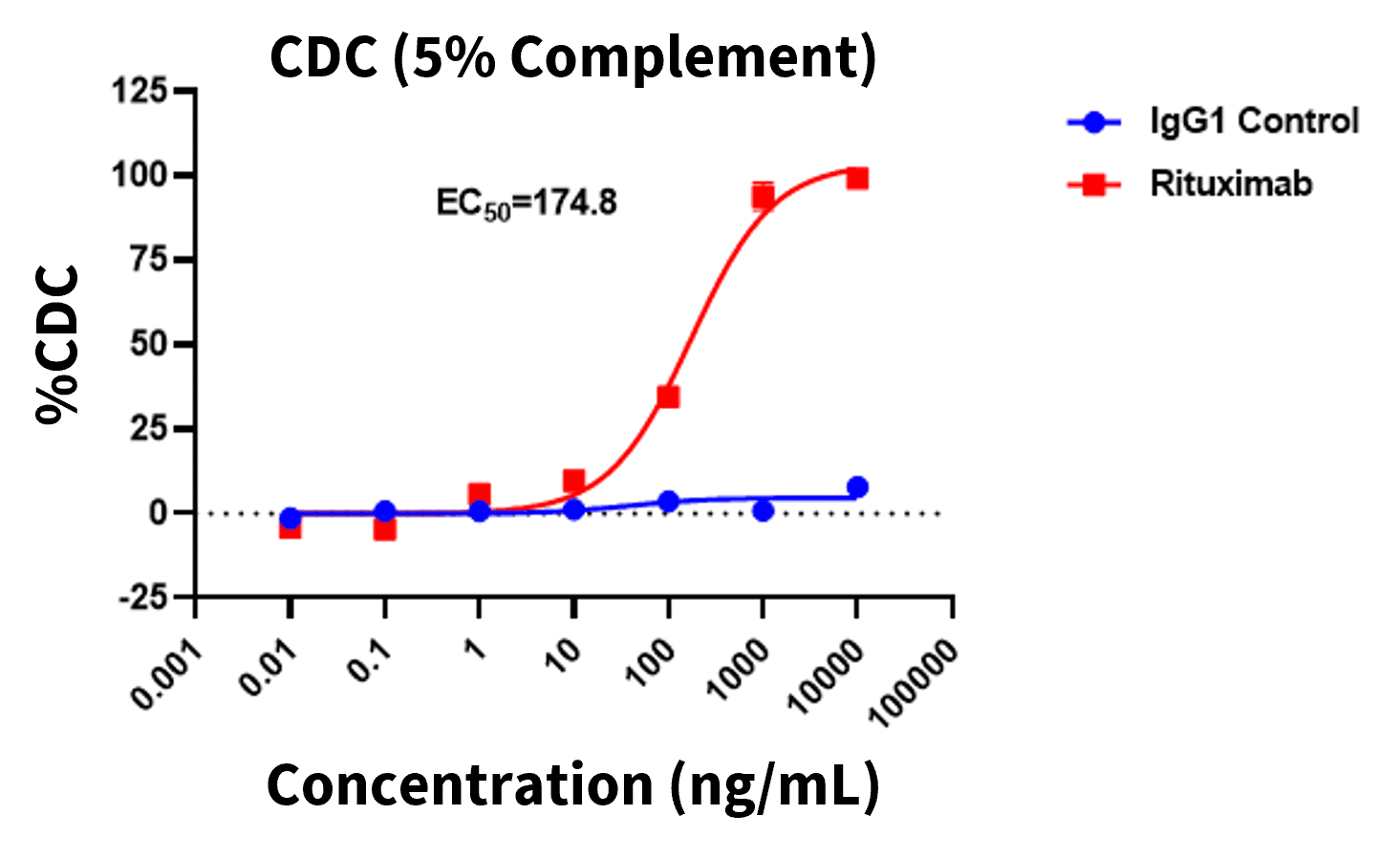
Daudi cells were incubated for 2 hours with either Rituximab or irrelevant IgG1 control in the presence of human serum. Complement-dependent cytotoxicity (CDC) induced by Rituximab was evaluated by Calcein AM positivity using flow cytometry.
-
T Cell Proliferation (Flow-based)
-
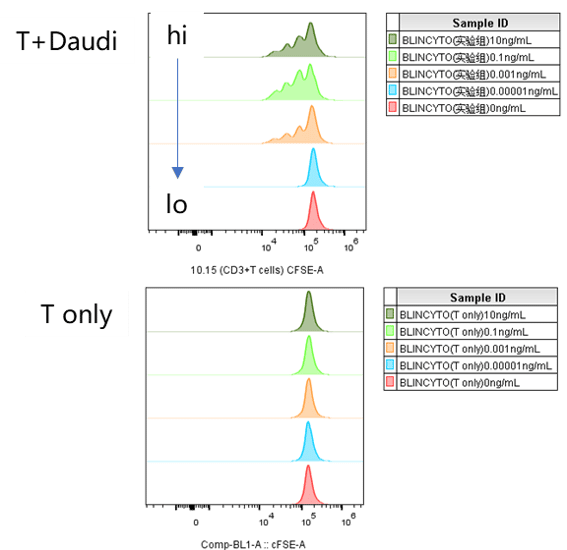
Daudi cells were incubated with CFSE-labeled CD3+ T cells and treated with various concentrations of Blinatumomab, which engages CD3 and CD20. T cell proliferation was assessed by flow cytometry after 3 days, which revealed more proliferation with higher doses of Blinatumomab, but only in the presence of Daudi cells.
-
Endocytosis/Internalization (Flow-based)
-
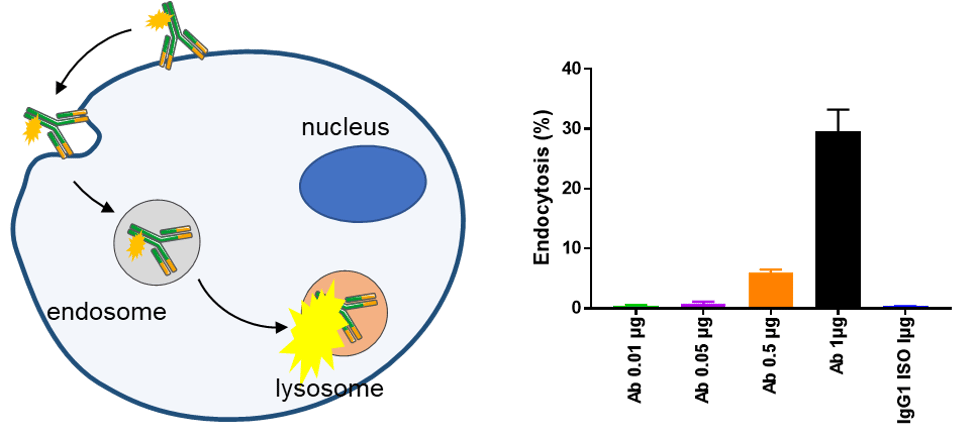
Murine MC38 tumor cells expressing human CLDN18.2 were incubated with a pH sensitive dye-labeled CLDN18.2 antibody for 24 hours. Internalization of the dye was assessed by flow cytometry, with the 1 µg dose significantly inducing endocytosis.
-
Endocytosis/Internalization (IncuCyte)
-
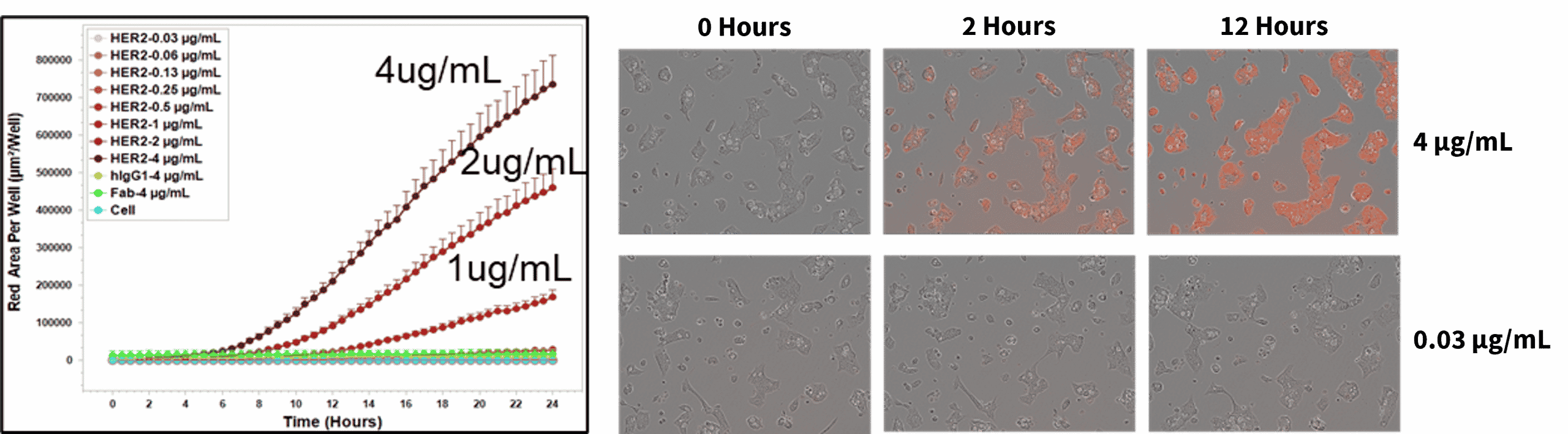
In addition to flow cytometry, internalization can also be measured in real time using the IncuCyte platform. In this study, NCI-N87 cells were treated with a pH-sensitive HER2 antibody. The fluorescent area per well (as shown by the static images) can be monitored in real time over a 24 hour period.



