Atopic Dermatitis Mouse Model Introduction
- Atopic dermatitis (AD) is a chronic skin disease that presents with itching, erythema and squamous lesions, and can be associated with cardiovascular disease, mental disorders (depression, anxiety, sleep disorders, etc.) and other diseases.
- Atopic dermatitis mouse models:
- Induced by enhanced skin sensitization using epithelial sensitive antigens, haptens, etc.
- Genetically engineered atopic dermatitis mouse model;
- Spontaneous atopic dermatitis mouse model.
- Biocytogen uses wild-type C57BL/6 mice and B-hIL4/hIL4RA double humanized mice on the C57BL/6 background to experimentally induce AD-like lesions via oxazolone (OXA) sensitization and continuous challenge or application of MC903. The resulting atopic dermatitis mouse models can be used for preclinical efficacy evaluation of therapeutic candidates.
Watch our webinar: Inflammatory Disease Modeling for Preclinical Studies
-
OXA-Induced Atopic Dermatitis Model
-
Experimental mouse strains: C57BL/6, BALB/c, 5-6 weeks old, female
Modeling reagent: Oxazolone (OXA)
Modeling method: Sensitization, day 0; Challenge, day 7-25
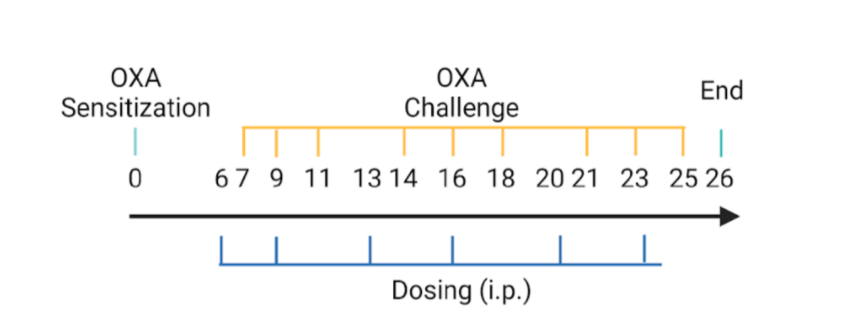
-
Clinical Assessment and Total Serum IgE Levels
-
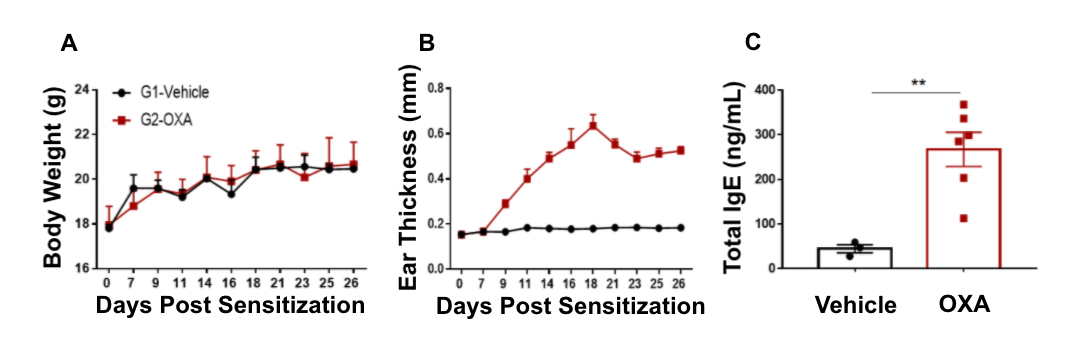
Atopic dermatitis was chemically induced in wild-type C57BL/6 mice by OXA sensitization and continuous challenge. (A) Body weight, (B) ear thickness, and (C) total serum IgE concentrations were measured in wild-type C57BL/6 mice exposed to vehicle control or OXA.
Ear thickness and serum IgE levels were significantly increased in wild-type C57BL/6 mice treated with OXA compared to vehicle control.
-
Histological Analysis
-
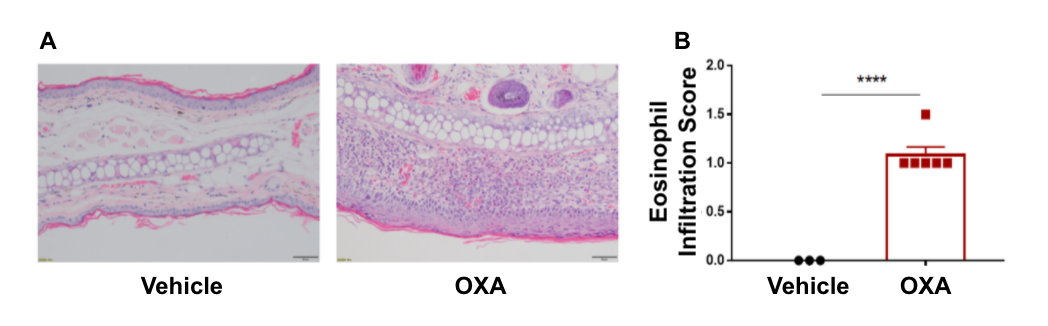
Ear skin pathology and lymphocyte infiltration analysis in a wild-type C57BL/6 atopic dermatitis mouse model. (A) Hematoxylin and eosin (H&E) staining of mouse ear tissue sections, and (B) eosinophil infiltration scores of the ear epidermis.
Wild-type C57BL/6 OXA-treated mice displayed varying degrees of stromal cell proliferation, thickening, hyperkeratosis with hypokeratosis, crust, mixed inflammatory cell infiltration in dermis and subcutaneous tissue and other atopic dermatitis-related pathological changes in the epidermis. Additionally, wild-type C57BL/6 OXA-treated mice had a significantly higher eosinophil infiltration score compared to wild-type C57BL/6 vehicle control-treated mice. These combined results demonstrate that wild-type C57BL/6 OXA-treated mice successfully induce atopic dermatitis-like pathology.
-
Dexamethasone Efficacy Analysis in Wild-Type-AD Mice
-
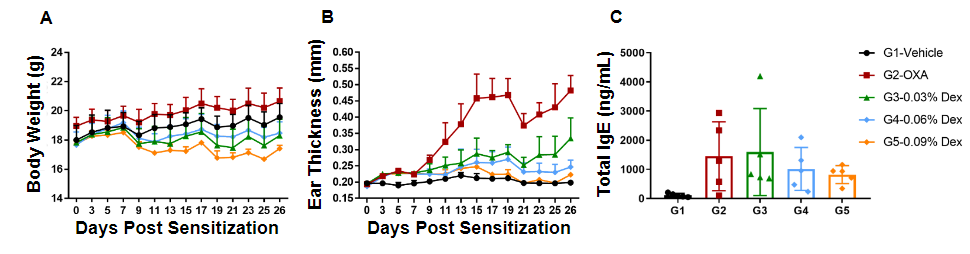 Efficacy of dexamethasone (Dex) was confirmed in a wild-type C57BL/6 atopic dermatitis mouse model. (A) Mouse body weight, (B) ear thickness, and (C) total serum IgE levels were measured in wild-type C57BL/6 mice exposed to vehicle control (G1), OXA alone (G2), or in combination with increasing concentrations of dexamethasone (G3-G5) as indicated in the dosing regimen (twice/week).
Efficacy of dexamethasone (Dex) was confirmed in a wild-type C57BL/6 atopic dermatitis mouse model. (A) Mouse body weight, (B) ear thickness, and (C) total serum IgE levels were measured in wild-type C57BL/6 mice exposed to vehicle control (G1), OXA alone (G2), or in combination with increasing concentrations of dexamethasone (G3-G5) as indicated in the dosing regimen (twice/week). Our results indicate that dexamethasone treatment significantly reduced ear thickness compared to wild-type C57BL/6 OXA-only mice. In particular, high dose of dexamethasone (0.09% Dex) in wild-type C57BL/6 OXA-treated mice completely relieved ear skin edema comparable to that of control levels, however it was also associated with significant weight loss compared to the control group. Lastly, medium and high doses of dexamethasone (0.06%, 0.09%) in wild-type C57BL/6 OXA-treated mice reduced the concentration of total serum IgE levels.
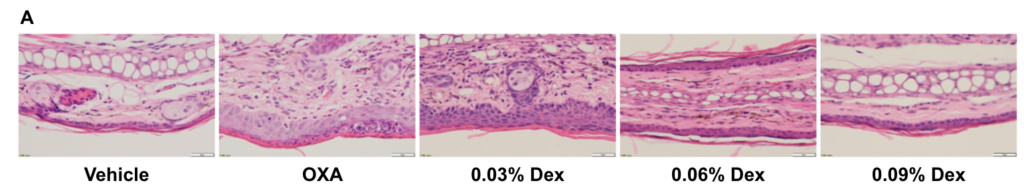
Efficacy of dexamethasone (Dex) was confirmed in a wild-type C57BL/6 atopic dermatitis mouse model. (A) Hematoxylin and eosin (H&E) staining of mouse ear tissue sections, and (B) eosinophil infiltration scores of the ear epidermis in wild-type C57BL/6 mice exposed to vehicle control (G1), OXA alone (G2), or in combination with increasing concentrations of dexamethasone (G3-G5) as indicated in the dosing regimen (twice/week).
Our results indicate that pathological changes related to atopic dermatitis, such as stromal cell proliferation, thickening, and mixed inflammatory cell infiltration were observed in the ear epidermis of wild-type C57BL/6 OXA-treated mice (G2). These observed phenotypes were significantly alleviated with dexamethasone administration in wild-type C57BL/6 OXA-treated mice(G3-G5) compared to OXA-alone, especially with high dose of dexamethasone (0.09%). Additionally, the eosinophil infiltration score in the ear epidermis was significantly lower with dexamethasone treatment. Overall, dexamethasone improved atopic dermatitis-related symptoms most notably at concentrations of 0.06% and 0.09%. These combined results indicate that the atopic dermatitis mouse model can be chemically induced by OXA and is a powerful tool for efficacy evaluation.
-
Human IL4 and IL4RA Expression in Humanized B-hIL4/hIL4RA Mice
-
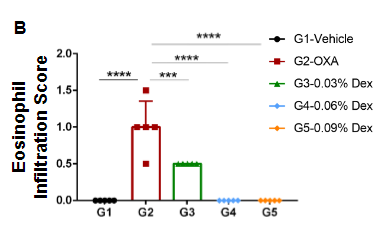
Species-specific IL4 and IL4RA expression analysis in homozygous humanized B-hIL4/hIL4RA mice. Following anti-CD3ɛ stimulation in vivo, (A) serum was collected from wild-type C57BL/6 (+/+) and homozygous B-hIL4/hIL4RA (H/H) mice and analyzed using a species-specific IL4 ELISA kit, while (B) splenocytes were isolated from wild-type C57BL/6 (+/+) and homozygous B-hIL4/hIL4RA (H/H) mice and analyzed by flow cytometry using a species-specific anti-IL4RA antibody.
These results suggest human IL4 and IL4RA is exclusively expressed in humanized B-hIL4/hIL4RA mice compared to wild-type C57BL/6 mice.
-
In vivo Anti-Human IL4RA Antibody Efficacy Evaluation Using Humanized B-hIL4/hIL4RA-AD Mice
-
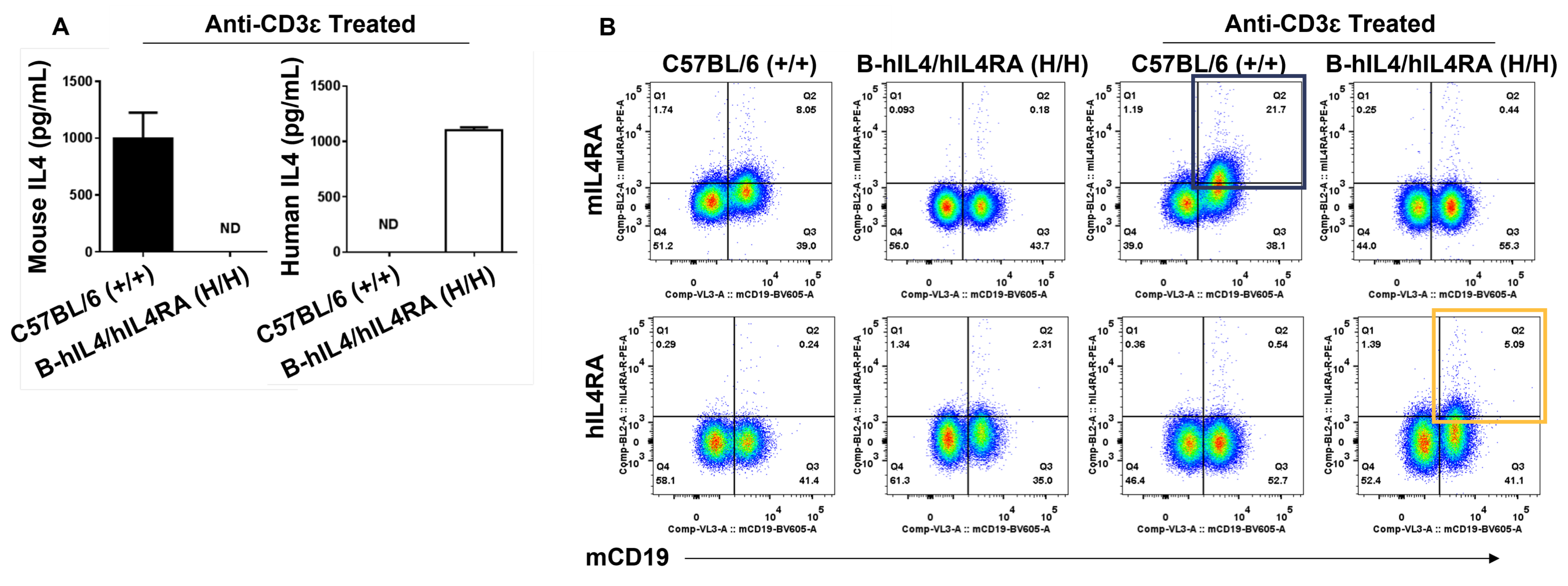
Anti-human IL4RA antibody efficacy was confirmed in humanized B-hIL4/hIL4RA mice with atopic dermatitis. (A) Mouse body weight, (B) ear thickness, and (C) total serum IgE levels were measured in humanized B-hIL4/hIL4RA mice exposed to vehicle control (G1), OXA-hIgG control (G2), or OXA in combination with increasing concentrations of an anti-human IL4RA antibody analog (dupilumab, G3-G6) as indicated in the dosing regimen (twice/week).
Our results indicate that dupilumab treatment improved ear thickness compared to B-hIL4/IL4RA OXA-hIgG treated mice. Additionally, high concentrations of dupilumab (25 mg/kg) in B-hIL4/IL4RA OXA-treated mice reduced the concentration of serum total IgE levels, altogether suggesting that ear thickness and total IgE levels are inversely correlated with dupilumab dose.
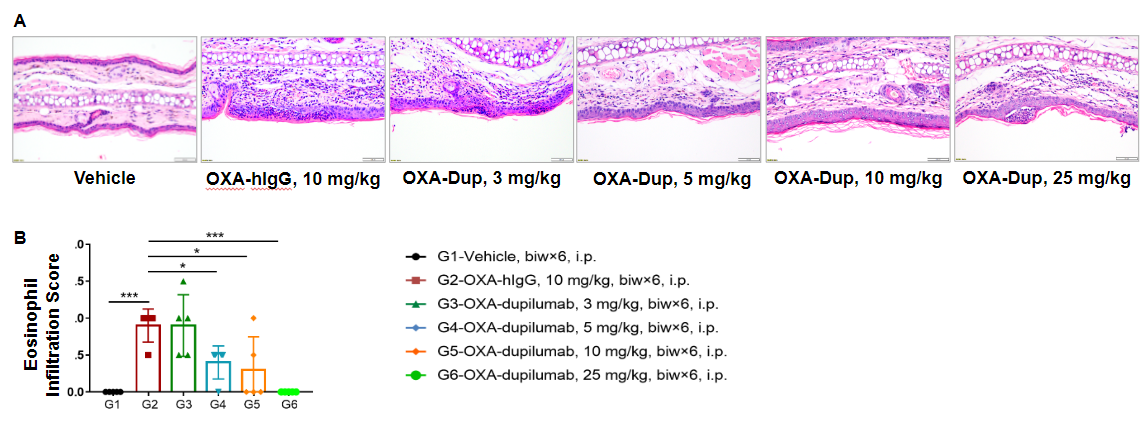
Efficacy of anti-human IL4RA antibody on skin and lymphocyte infiltration in the ear of B-hIL4/hIL4RA mice with experimental atopic dermatitis. (A) Hematoxylin and eosin (H&E) staining of mouse ear tissue sections, and (B) eosinophil infiltration scores of the ear epidermis in B-hIL4/hIL4RA mice exposed to vehicle control (G1), OXA-hIgG control (G2), or OXA in combination with increasing concentrations of an anti-human IL4RA antibody analog (dupilumab, G3-G6) as indicated in the dosing regimen (twice/week).
Our results indicate that pathological changes related to atopic dermatitis, such as stromal cell proliferation, thickening, and mixed inflammatory cell infiltration were observed in the ear epidermis of B-hIL4/hIL4RA OXA-hIgG treated mice (G2). These observed phenotypes were significantly alleviated with dupilumab administration in B-hIL4/hIL4RA OXA-treated mice (G3-G6) compared to OXA-hIgG, especially with high doses of dupilumab (25 mg/kg). Additionally, the eosinophil infiltration score in the ear epidermis was significantly lower with high doses of dupilumab treatment. Overall, dupilumab improved atopic dermatitis-related symptoms in B-hIL4/hIL4RA OXA-treated mice, indicating that B-hIL4/hIL4RA mice are a powerful preclinical model for in vivo efficacy evaluation of anti-human IL4RA antibodies.
-
Human TSLP and TSLPR Expression in Humanized B-hTSLP/hTSLPR Mice
-
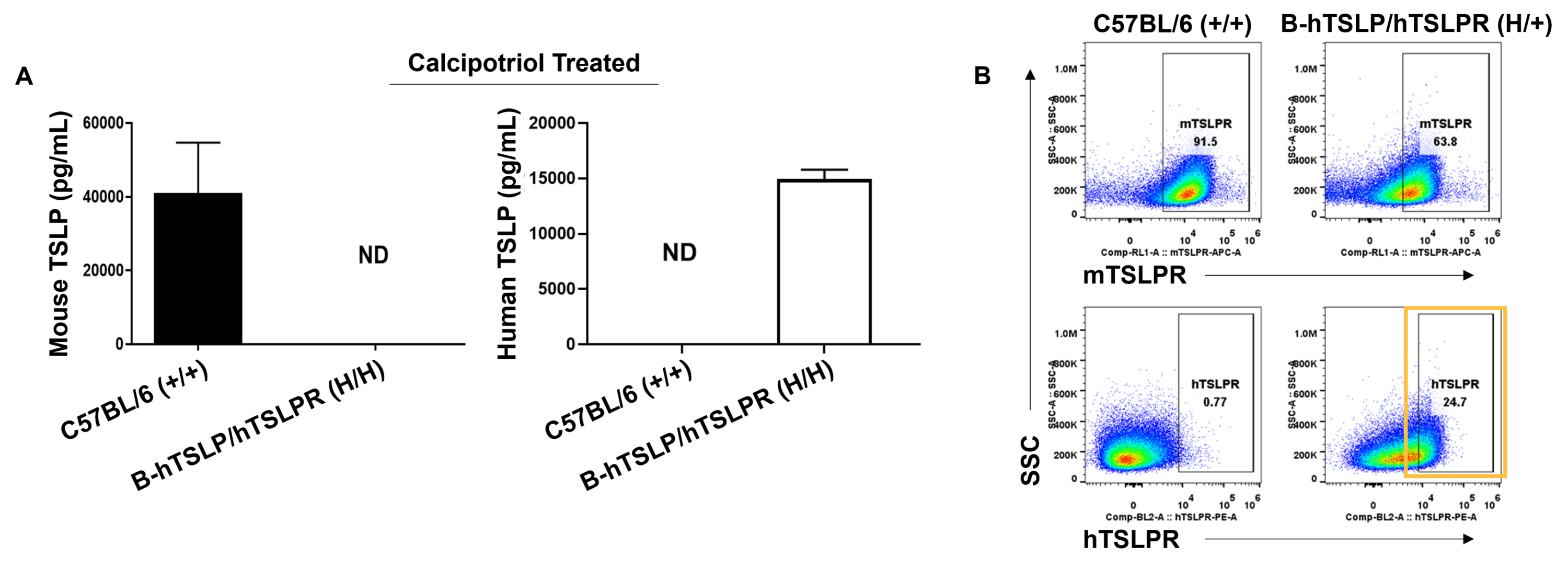
Species-specific TSLP and TSLPR expression analysis in humanized B-hTSLP/hTSLPR mice. (A) Calcipotriol was first topically applied on the ears of wild-type and homozygous B-hTSLP/hTSLPR mice for 7 days. Ear grinding supernatant was collected from mice and analyzed by ELISA using a species-specific TSLP ELISA kit. Mouse TSLP was detected in wild-type mice, while human TSLP was exclusively detected in B-hTSLP/hTSLPR mice. (B) Macrophages isolated from wild-type and heterozygous B-hTSLP/hTSLPR mice were analyzed by flow cytometry using species-specific anti-TSLPR antibodies. Mouse TSLPR was detected in wild-type and B-hTSLP/hTSLPR mice, while human TSLPR was exclusively detected in B-hTSLP/hTSLPR mice.
These results suggest human TSLP and TSLPR proteins are exclusively expressed in humanized B-hTSLP/hTSLPR mice compared to wild-type C57BL/6 mice.
-
In vivo Anti-Human TSLP Antibody Efficacy Evaluation Using Humanized B-hTSLP/hTSLPR-AD Mice
-

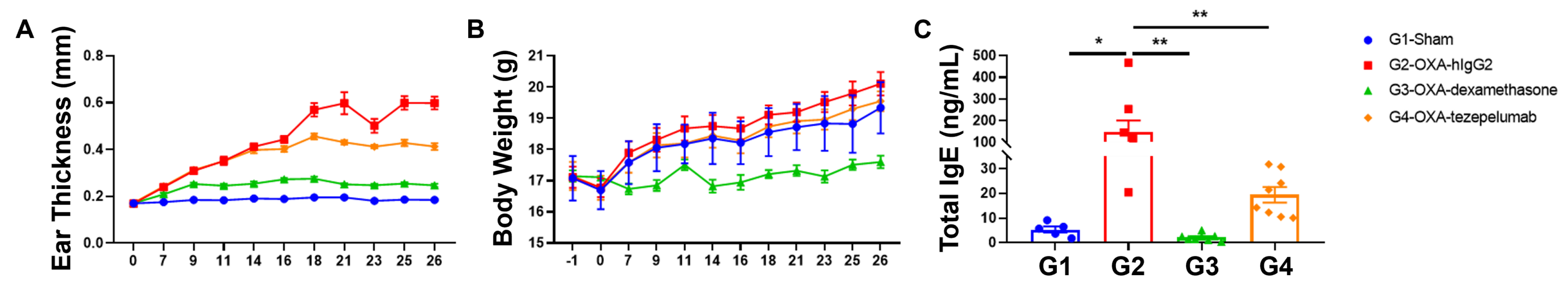
Anti-human TSLP antibody efficacy was confirmed in humanized B-hTSLP/hTSLPR mice with atopic dermatitis. (A) Ear thickness, (B) body weight, and (C) serum total IgE levels were measured in humanized B-hTSLP/hTSLPR mice exposed to vehicle control (G1), OXA-hIgG control (G2), or OXA in combination with either dexamethasone (G3) or an anti-human TSLP antibody analog (tezepelumab, G4) as indicated in the model build diagram.
Tezepelumab administration in OXA-treated B-hTSLP/hTSLPR mice improved ear thickness and reduced the concentration of serum total IgE levels compared with the oxazolone plus isotype control group.
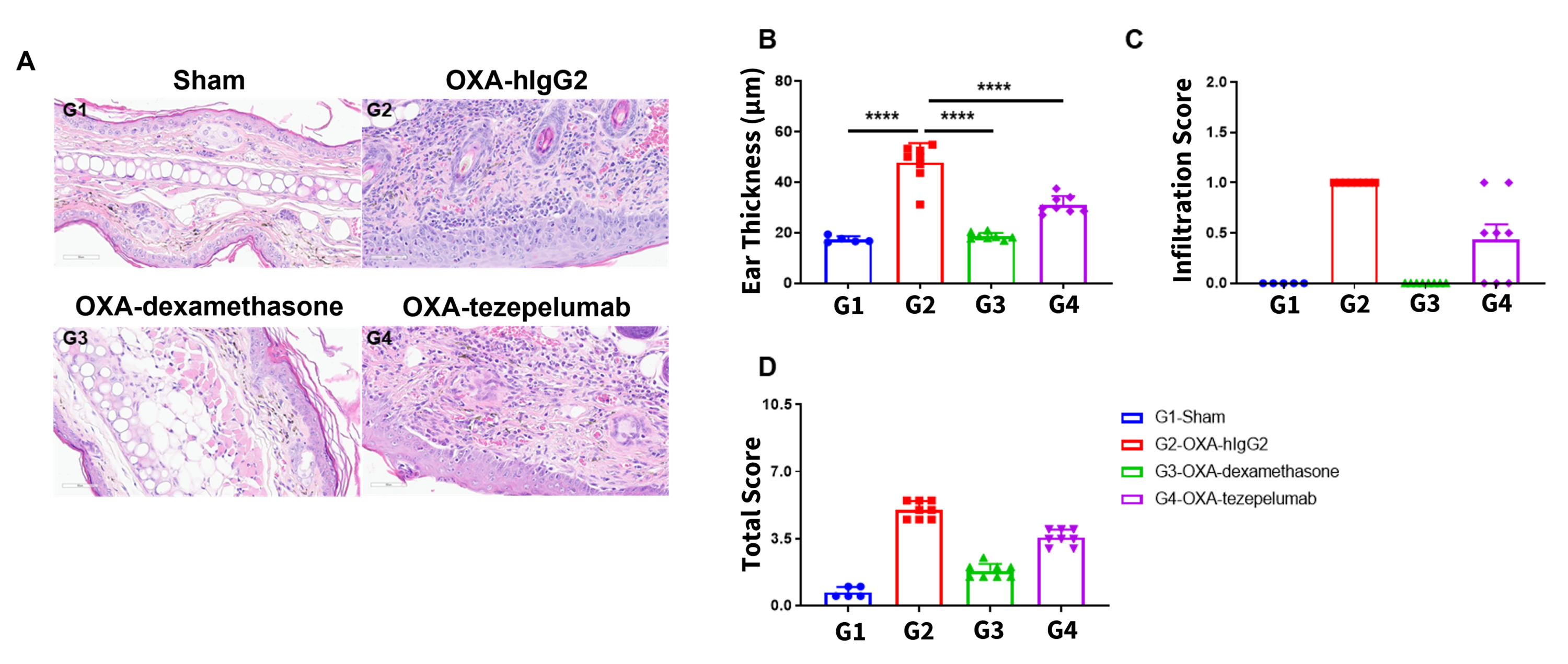
Efficacy of anti-human TSLP antibody on skin and eosinophil infiltration in the ear of B-hTSLP/hTSLPR mice with experimental atopic dermatitis. (A, B) Hematoxylin and eosin (H&E) staining of mouse ear tissue sections, (C) eosinophil infiltration scores, (D) and total score of the ear epidermis in B-hTSLP/hTSLPR mice exposed to vehicle control (G1), OXA-hIgG control (G2), or OXA in combination with either dexamethasone (G3) or an anti-human TSLP antibody analog (tezepelumab, G4) as indicated in the model build diagram.
Tezepelumab treatment alleviated some of the pathological changes related to OXA-induced atopic dermatitis such as ear thickness and eosinophil infiltration. Together these results indicate that our B-hTSLP/hTSLPR mice provide a promising preclinical model for in vivo efficacy evaluation of anti-human TSLP antibodies.
-
MC903-Induced Atopic Dermatitis Model
-
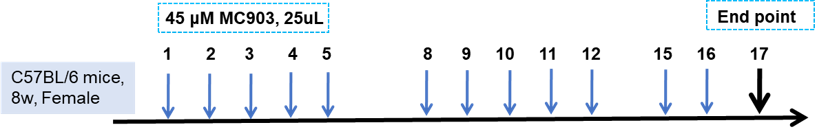
Experimental mouse strains: C57BL/6, 8-weeks-old, female
Modeling reagent: MC903
Modeling method: MC903 application on right ear
Detection indicator: Ear thickness, total IgE, H&E staining
-
Clinical Assessment and Total Serum IgE Levels
-
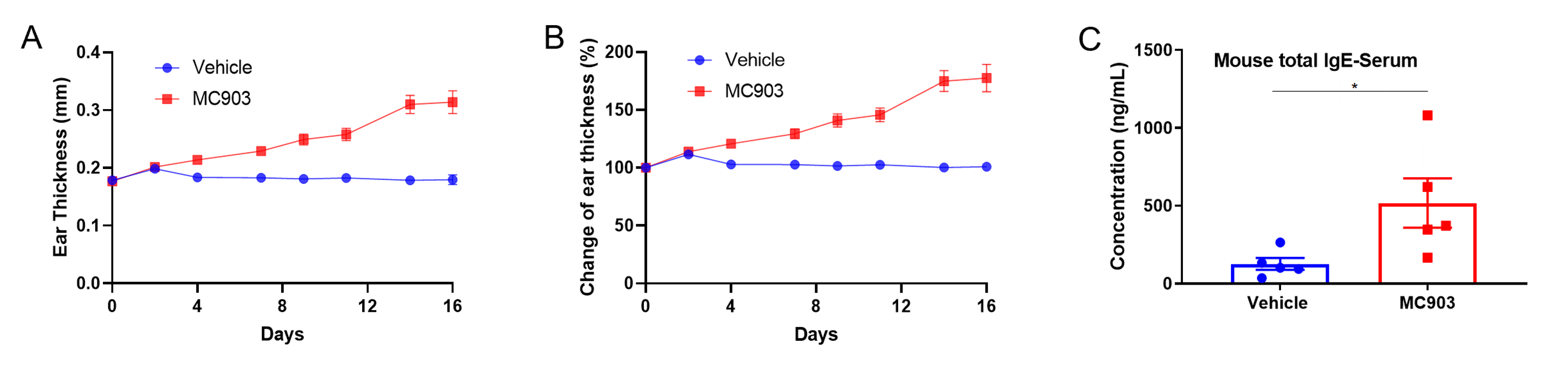
Atopic dermatitis was induced by MC903 in wild-type C57BL/6 mice. (A-B) Change in ear thickness. (C) Serum total IgE levels. Ear thickness and serum IgE were significantly increased in the MC903-treated group. Values are expressed as mean ± SEM. *p<0.05.
-
Histological Analysis
-
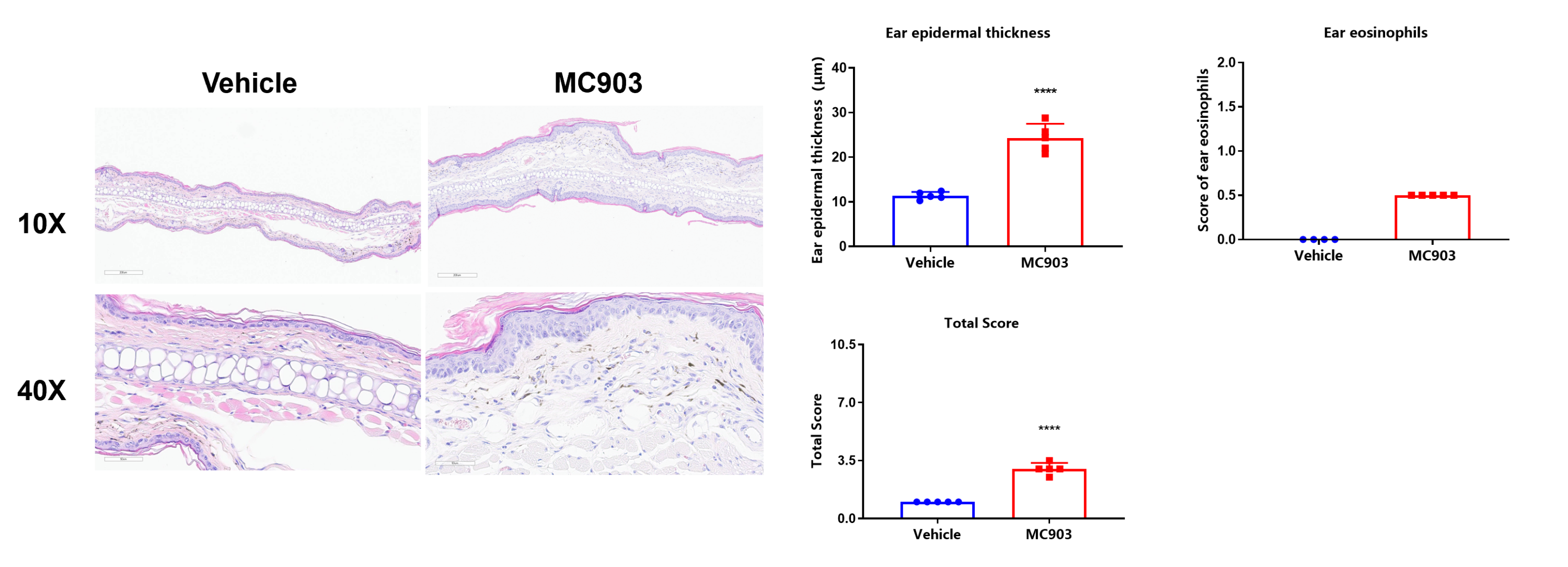
Histological analysis and pathological scores in MC903-induced atopic dermatitis mice. Compared with the vehicle group, MC903-treated mice showed epidermal hyperplasia, epidermal hyperkeratosis, severe skin erosions, and intra-epidermal inflammatory cell infiltration in the superficial dermis. Values are expressed as mean ± SEM. ****p<0.0001.



