Psoriasis Model Introduction
Psoriasis is a type of skin disease characterized by hyperproliferation of the epidermal keratinocytes. It is thought to be mediated by cells and molecules of both the innate and adaptive immune systems, as a result of a combination of genetic, epigenetic, and environmental influences. The most commonly used animal model is the imiquimod (IMQ)-induced skin lesion and skin inflammation, which phenotypically resembles human psoriasis.
The degree of imiquimod-induced skin injury in this murine psoriasis model, with symptoms of skin rashes and peeling in mice, can be monitored through a standard clinical score system. The cutaneous epidermis is thickened. Parakeratosis and inflammatory infiltration of leukocytes predominantly into dermis can be seen histopathologically. The IL-23/IL-17 axis is believed to be particularly important in psoriasis pathogenesis due to its pro-inflammatory effects. IL-17 cytokine level is elevated in the imiquimod-induced psoriasis model.
Biocytogen provides a humanized imiquimod-induced psoriasis model for your psoriasis drug discovery applications. In particular, we generated B-hIL17A mice where the human IL-17A gene is knocked-in and replaces the mouse IL-17A gene. This genetically humanized mouse psoriasis model allows for for convenient testing of therapeutics targeting human IL-17A.
Watch the webinar: Investigating Inflammatory Disease using Humanized Cytokine Mice
-
Psoriasis Model Induction
-
Experimental mouse strains: C57BL/6 or BALB/c, 8-week-old, female
Modeling reagent: Imiquimod (IMQ)
Modeling method: Topically applicated on dorsal skin consecutively for 5-8 days (depending on mouse strain).
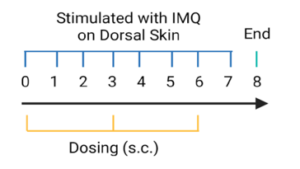
Psoriasis Mouse Model Criteria:
- Clinical Assessment: body weight, scaling and erythema of back skin
- Thickness of epidermal layer
- Pathological analysis using H&E staining
-
Clinical Assessment and Pathological Analysis of IMQ-Induced Psoriasis in C57BL/6 Mice
-
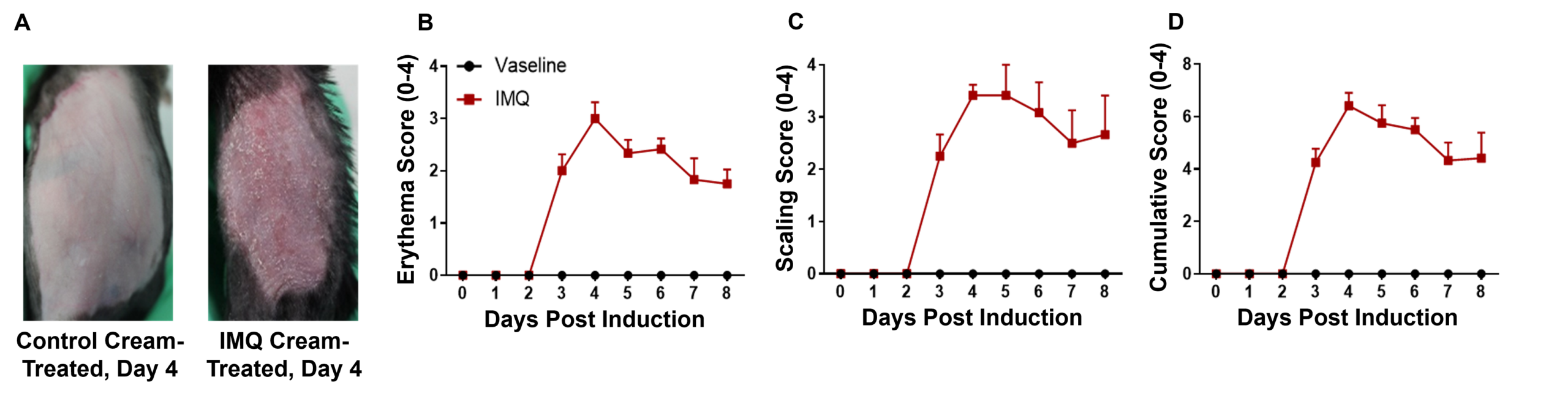 Psoriasis-like skin inflammation was induced in wild-type C57BL/6 mice by IMQ. (A) Phenotypical presentation of mouse dorsal skin after 4 days of treatment. (B,C) Daily dorsal scoring for erythema and scaling on a scale from 0 to 4. (D) Cumulative score (erythema and scaling) is also depicted. While wild-type C57BL/6 mice treated with control cream did not show any sign of inflammation, phenotypic features of IMQ-induced skin inflammation was visible and increased in severity up to day 4.
Psoriasis-like skin inflammation was induced in wild-type C57BL/6 mice by IMQ. (A) Phenotypical presentation of mouse dorsal skin after 4 days of treatment. (B,C) Daily dorsal scoring for erythema and scaling on a scale from 0 to 4. (D) Cumulative score (erythema and scaling) is also depicted. While wild-type C57BL/6 mice treated with control cream did not show any sign of inflammation, phenotypic features of IMQ-induced skin inflammation was visible and increased in severity up to day 4.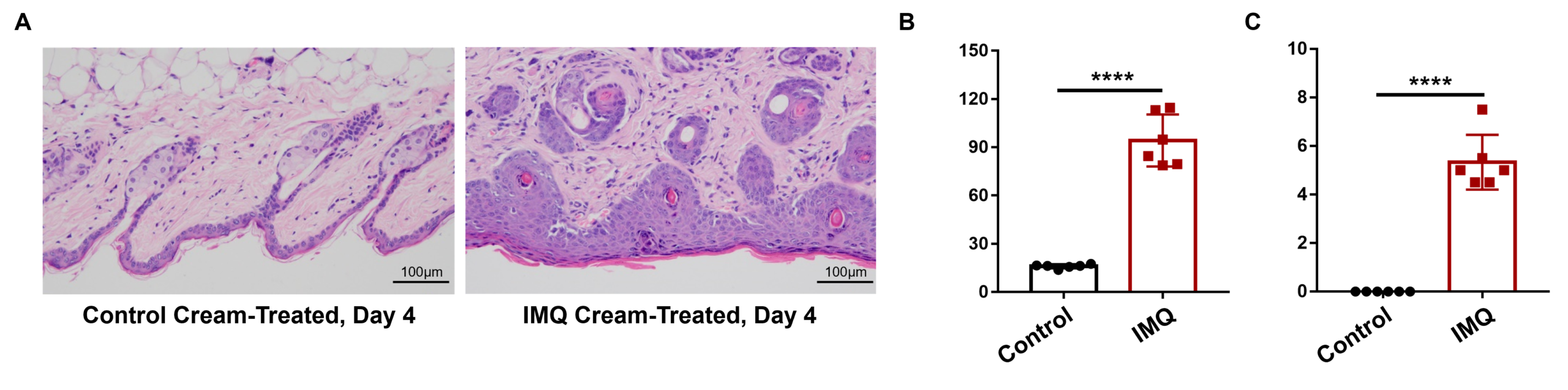 IMQ application induces keratinocyte proliferation and inflammatory cell infiltration. (A) Hematoxylin and eosin (H&E) staining, (B) epidermal thickness measurement, and (C) histology score on a scale from 0 to 11 of the dorsal skin from wild-type C57BL/6 mice treated with either control or IMQ cream. Compared to wild-type C57BL/6 mice treated with control cream, IMQ-treated mice showed significantly increased epidermal thickness, in addition to pathological observations that indicate IMQ resulted in psoriasis-like skin lesions and inflammation.
IMQ application induces keratinocyte proliferation and inflammatory cell infiltration. (A) Hematoxylin and eosin (H&E) staining, (B) epidermal thickness measurement, and (C) histology score on a scale from 0 to 11 of the dorsal skin from wild-type C57BL/6 mice treated with either control or IMQ cream. Compared to wild-type C57BL/6 mice treated with control cream, IMQ-treated mice showed significantly increased epidermal thickness, in addition to pathological observations that indicate IMQ resulted in psoriasis-like skin lesions and inflammation. -
Dexamethasone Efficacy Validation in C57BL/6-Psoriasis Mice
-
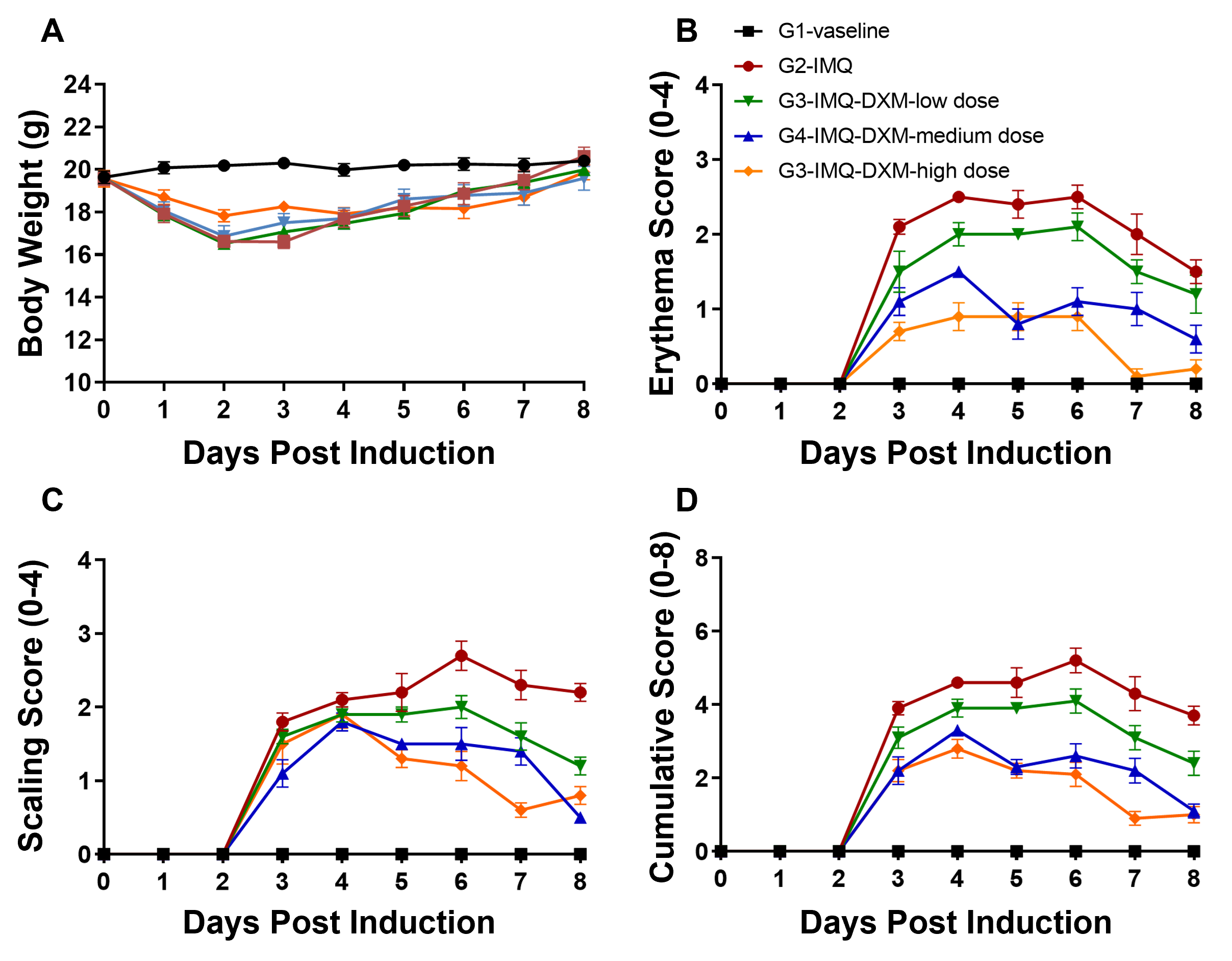 Dose-dependent effects of dexamethasone (DXM) in IMQ-induced wild-type C57BL/6 psoriasis mice. (A) Body weight, (B,C) daily dorsal scoring for erythema and scaling on a scale from 0 to 4, and (D) cumulative score (erythema and scaling) were measured in wild-type C57BL/6 mice exposed to control cream (G1), IMQ cream (G2), or IMQ in combination with increasing concentrations of dexamethasone (G3-G5). Phenotypic features of IMQ-induced skin inflammation were visible and increased in severity. High doses of dexamethasone alleviated pathological changes associated with IMQ-induced psoriasis, without negatively impacting body weight.
Dose-dependent effects of dexamethasone (DXM) in IMQ-induced wild-type C57BL/6 psoriasis mice. (A) Body weight, (B,C) daily dorsal scoring for erythema and scaling on a scale from 0 to 4, and (D) cumulative score (erythema and scaling) were measured in wild-type C57BL/6 mice exposed to control cream (G1), IMQ cream (G2), or IMQ in combination with increasing concentrations of dexamethasone (G3-G5). Phenotypic features of IMQ-induced skin inflammation were visible and increased in severity. High doses of dexamethasone alleviated pathological changes associated with IMQ-induced psoriasis, without negatively impacting body weight.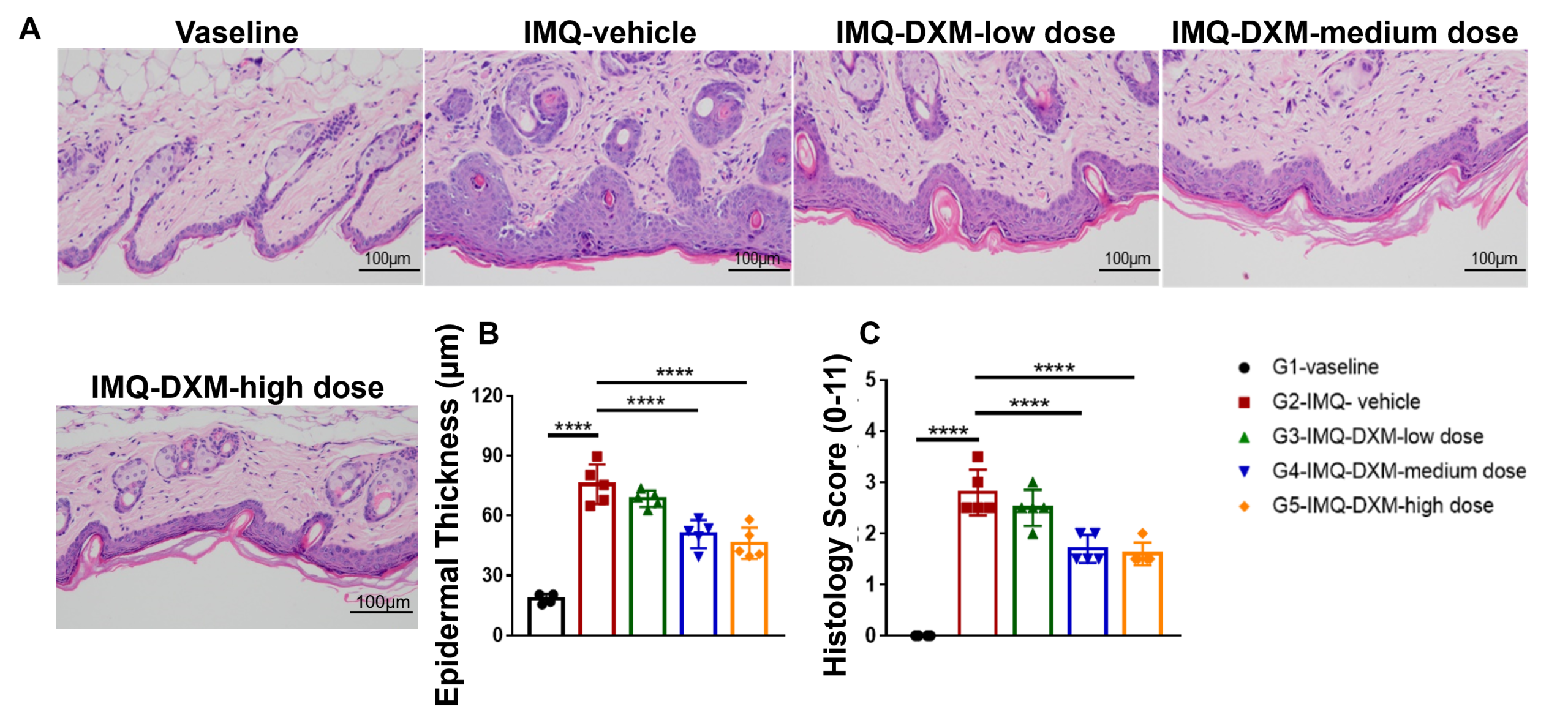 Dose-dependent effects of dexamethasone (DXM) on keratinocyte proliferation and inflammatory cell infiltration in IMQ induced wild-type C57BL/6 psoriasis mice. (A) Hematoxylin and eosin (H&E) staining, (B) epidermal thickness measurement, and (C) histology score on a scale from 0 to 11 of the dorsal skin from wild-type C57BL/6 mice exposed to control cream (G1), IMQ cream (G2), or IMQ in combination with increasing concentrations of dexamethasone (G3-G5). Dexamethasone alleviated psoriasis-like injury and skin inflammation in IMQ-treated wild-type C57BL/6 mice in a dose-dependent manner.
Dose-dependent effects of dexamethasone (DXM) on keratinocyte proliferation and inflammatory cell infiltration in IMQ induced wild-type C57BL/6 psoriasis mice. (A) Hematoxylin and eosin (H&E) staining, (B) epidermal thickness measurement, and (C) histology score on a scale from 0 to 11 of the dorsal skin from wild-type C57BL/6 mice exposed to control cream (G1), IMQ cream (G2), or IMQ in combination with increasing concentrations of dexamethasone (G3-G5). Dexamethasone alleviated psoriasis-like injury and skin inflammation in IMQ-treated wild-type C57BL/6 mice in a dose-dependent manner. -
Human IL17A and IL17F Expression in Humanized B-hIL17A/hIL17F Mice
-
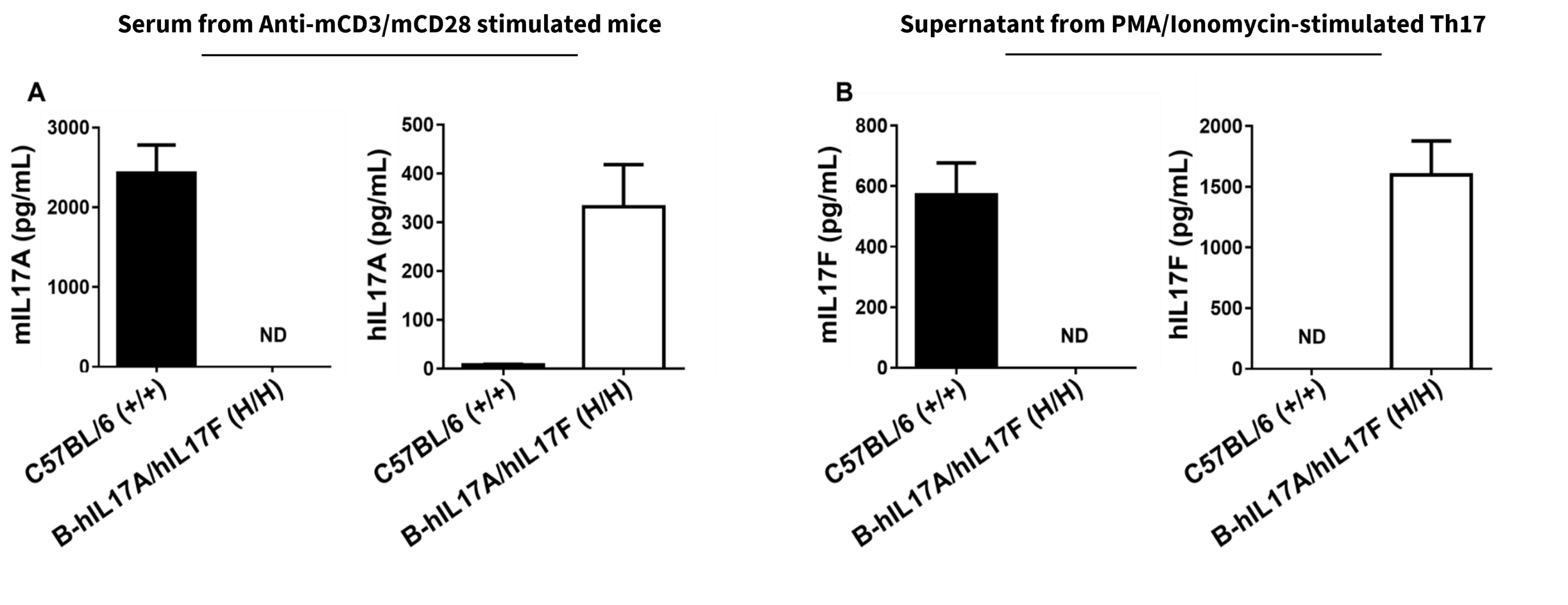
Species-specific IL17A and IL17F expression in homozygous humanized B-hIL17A/hIL17F mice. (A) Following anti-mCD3ɛ and anti-mCD28 antibody stimulation in vivo, serum was collected from wild-type C57BL/6 (+/+) and homozygous B-hIL17A/hIL17F (H/H) mice and analyzed using a species-specific IL17A ELISA kit. (B) Naïve CD4+ T cells were sorted from splenocytes isolated from wild-type C57BL/6 and homozygous B-hIL17A/IL17F mice and induced into Th17 cells. Following PMA and Ionomycin stimulation, Th17 cell culture supernatant was collected and analyzed using a species-specific IL17F ELISA kit. These results indicate human IL17A and IL17F is exclusively expressed in humanized B-hIL6/hIL6R mice compared to wild-type C57BL/6 mice.
-
Bimekizumab Efficacy Validation in Humanized B-hIL17A/hIL17F-Psoriasis Mice
-
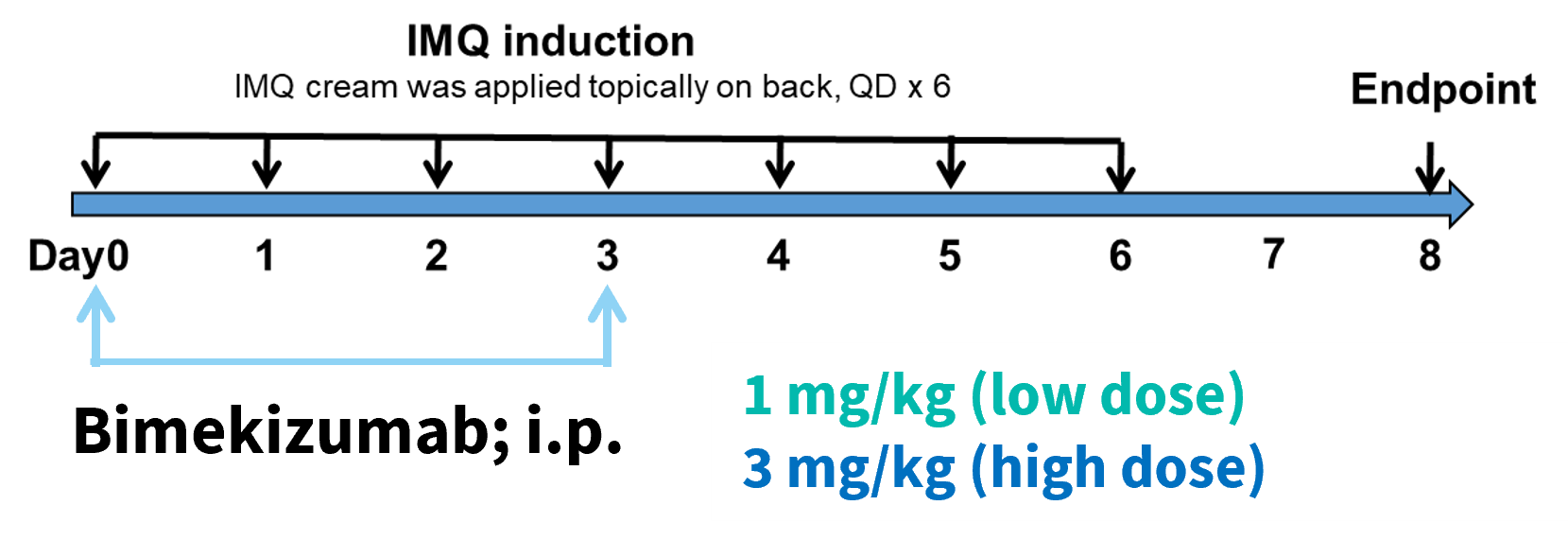
Beginning on day 0, Bimekizumab, an anti-human IL-17 antibody, was intermittently dosed at both low and high doses.
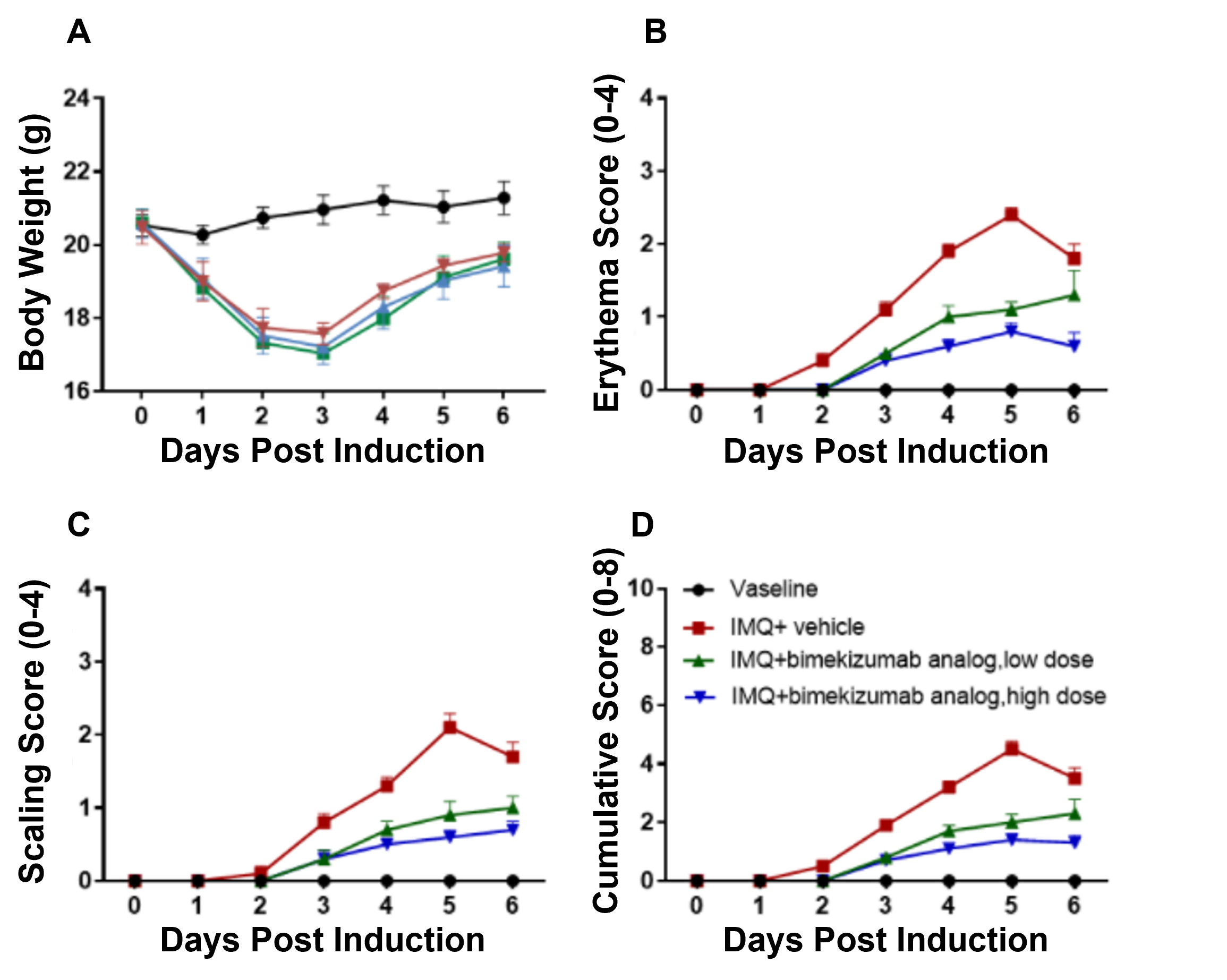 Dose-dependent effects of bimekizumab in IMQ-induced humanized B-hIL17A/hIL17F-psoriasis mice. (A) Body weight, (B,C) daily dorsal scoring for erythema and scaling on a scale from 0 to 4, and (D) cumulative score (erythema and scaling) were measured in humanized B-hIL17A/hIL17F mice exposed to control cream, IMQ cream, or IMQ in combination with different doses of an anti-human IL17A and IL17F antibody: bimekizumab (synthesized in-house). Phenotypic features of IMQ-induced skin inflammation was visible and increased in severity. High doses of bimekizumab alleviated pathological changes associated with IMQ-induced psoriasis.
Dose-dependent effects of bimekizumab in IMQ-induced humanized B-hIL17A/hIL17F-psoriasis mice. (A) Body weight, (B,C) daily dorsal scoring for erythema and scaling on a scale from 0 to 4, and (D) cumulative score (erythema and scaling) were measured in humanized B-hIL17A/hIL17F mice exposed to control cream, IMQ cream, or IMQ in combination with different doses of an anti-human IL17A and IL17F antibody: bimekizumab (synthesized in-house). Phenotypic features of IMQ-induced skin inflammation was visible and increased in severity. High doses of bimekizumab alleviated pathological changes associated with IMQ-induced psoriasis.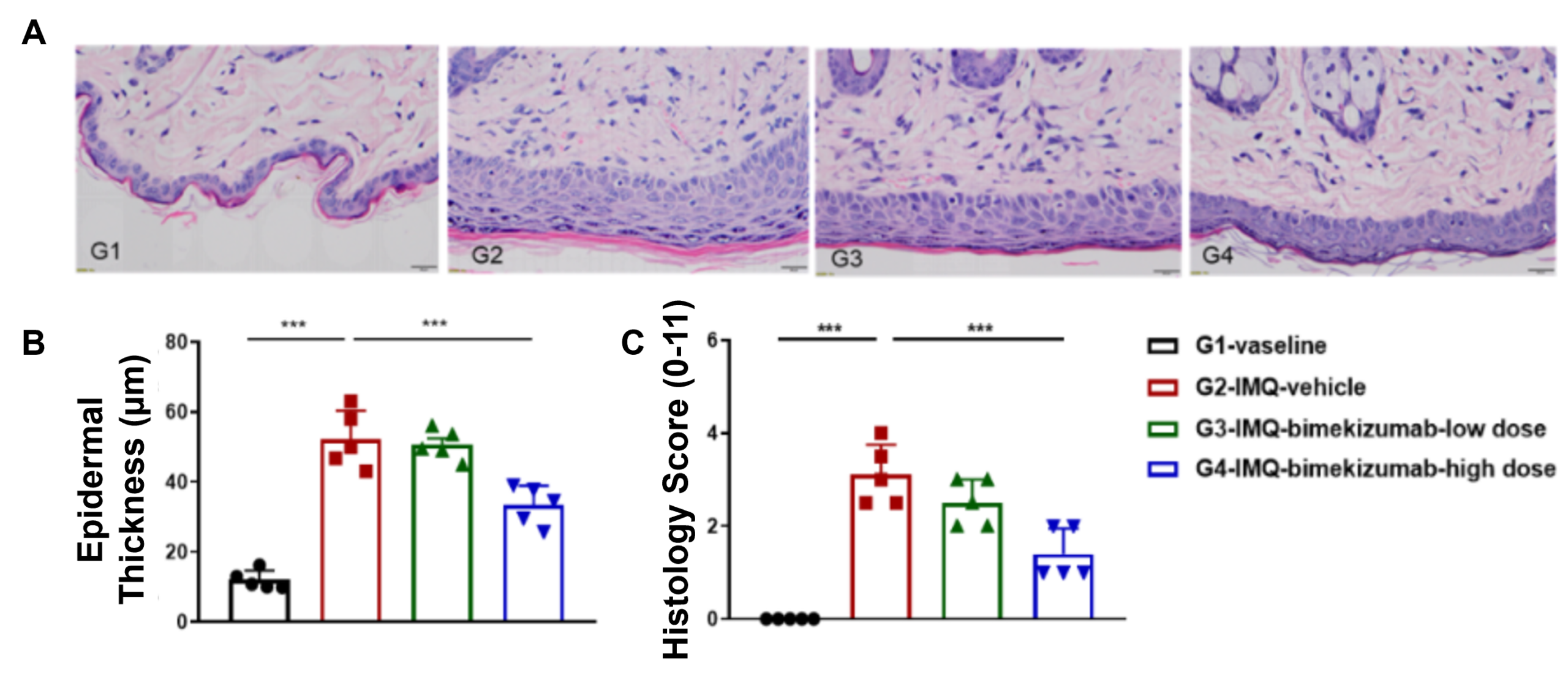 Dose-dependent effects of bimekizumab on keratinocyte proliferation and inflammatory cell infiltration in IMQ-induced psoriasis-like skin lesions in B-hIL17A/hIL17F mice. (A) Hematoxylin and eosin (H&E) staining, (B) epidermal thickness measurement, and (C) histology score on a scale from 0 to 11 of the dorsal skin from humanized B-hIL17A/hIL17F mice exposed to control cream, IMQ cream, or IMQ in combination with different doses of bimekizumab (synthesized in-house). Bimekizumab treatment significantly decreased psoriasis-like skin lesions in IMQ-treated humanized B-hIL17A/IL17F mice. Altogether this data suggests the B-hIL17A/hIL17F mice are a powerful preclinical model for in vivo evaluation of anti-human IL17A and IL17F antibodies.
Dose-dependent effects of bimekizumab on keratinocyte proliferation and inflammatory cell infiltration in IMQ-induced psoriasis-like skin lesions in B-hIL17A/hIL17F mice. (A) Hematoxylin and eosin (H&E) staining, (B) epidermal thickness measurement, and (C) histology score on a scale from 0 to 11 of the dorsal skin from humanized B-hIL17A/hIL17F mice exposed to control cream, IMQ cream, or IMQ in combination with different doses of bimekizumab (synthesized in-house). Bimekizumab treatment significantly decreased psoriasis-like skin lesions in IMQ-treated humanized B-hIL17A/IL17F mice. Altogether this data suggests the B-hIL17A/hIL17F mice are a powerful preclinical model for in vivo evaluation of anti-human IL17A and IL17F antibodies.



