Antibody Screening Using Syngeneic Mouse Tumor Models
Many antibodies that target human immune checkpoints do not cross-react well to murine counterparts, making it difficult to test antibody efficacy in vivo. Biocytogen’s humanized models provide an excellent solution where the human extracellular domain replaces the mouse counterpart, thus allowing direct testing of anti-human antibodies in mice for in vivo efficacy assessments. At Biocytogen, many of our humanized mice are assessed for the following features, allowing for pre-clinical validation of novel antibodies:
- Expression of the humanized portion of the gene by RT-PCR & FACS whenever possible
- Lymphocyte development & cell subtypes in the spleen, thymus, lymph nodes at frequencies comparable to those observed in wildtype C57BL/6 mice
Similarly, Biocytogen has engineered several syngeneic models carrying humanized immune checkpoint genes or other tumor-associated antigens. These syngeneic models can be engrafted into our humanized immune checkpoint mice for robust anti-tumor efficacy studies. Many of our mouse models also carry multiple humanized genes, allowing for the evaluation of novel combination therapies for anti-tumor efficacy studies. View specific case studies in humanized CD3E mice below.
-
Humanized CD3ε Mouse Model
-
Biocytogen’s B-hCD3ε mice have been engineered to express the human extracellular domain of CD3ε in place of the mouse counterpart, thus allowing direct testing of anti-human CD3 antibodies (including bispecific anti-human CD3 antibodies) in mice for in vivo efficacy assessments. Our gene editing team used conventional ES/HR technology to knock-in human CD3ε genomic DNA from 3’ part of exon 2 to 5’ of exon7 to replace the corresponding mouse exons 2-6.
These B-hCD3ε mice have the following features, allowing pre-clinical validation for bispecific antibodies:
- Human CD3ε are expressed in homozygous B-hCD3ε mice whereas mouse CD3ε+ cells are absent.
- T cell development is normal in B-hCD3ε homozygous mice.
- T cell subtypes in the spleen, thymus, lymph nodes, and PBMC in B-hCD3ε mice are comparable to those in wildtype C57BL/6 mice.
- Anti-mouse PD-1 antibody significantly represses the growth of MC38 tumor cells engrafted in B-hCD3ε mice, suggesting that T cells in B-hCD3ε mice are functional.
- Treatment of B-hCD3ε mice with anti-human CD3ε antibody OKT3 leads to T cell depletion, suggesting functional human CD3ε.
- Normal in vitro T cell proliferation and cytokine production are found in anti-hCD3ε antibody treated humanized B-hCD3ε mice.
-
In Vivo Bispecific Antibody Efficacy Evaluation
-
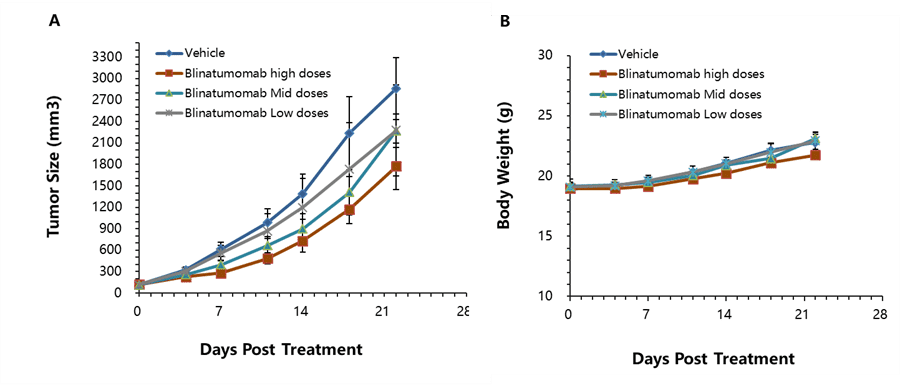
Murine colon cancer MC38-hCD19 cells were subcutaneously implanted into B-hCD3ε mice. Mice were divided into control and treatment group dose(n=6) when the tumor size was approximately 150±50 mm3. High doses of hCD3ε antibody (Blinatumomab;in-house analog) significantly inhibited tumor growth, confirming that the B-hCD3ε mouse model is a powerful tool for in vivo anti-hCD3ε based bi-specific antibody efficacy evaluation. (A) Tumor average volume ± SEM, (B) Mice average weight ±SEM.
-
T Cell Assessments
-
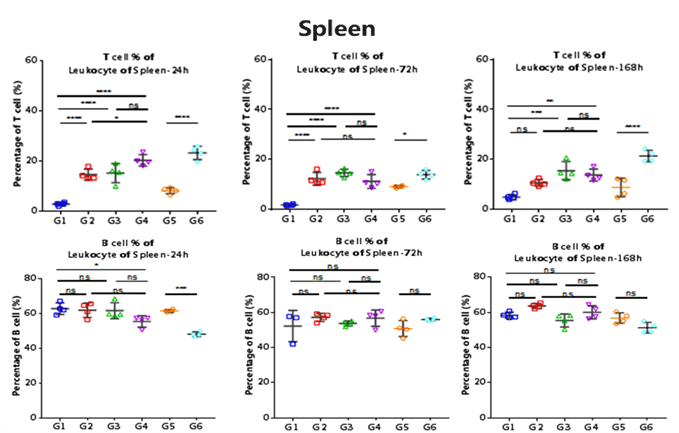
Strains of Mice Group Treatment B-hCD3ε G1 hCD3 Antibody(OKT3) B-hCD3ε G2 CD3/CD19 Bispecific Antibody B-hCD3ε G3 Blinatuomomab(analog) B-hCD3ε G4 PBS C57BL/6 G5 mCD3 antibody (145-2C11) C57BL/6 G6 PBS The ratio of T cells in spleen and blood were analyzed at 24h, 72h and 168h by flow cytometry.
-
Assessments for Activation-Induced Cell Death
-
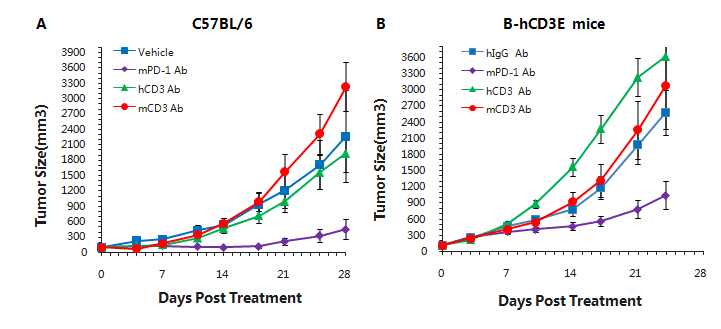
Murine colon cancer MC38 cells were subcutaneously implanted into C57BL/6(A) and B-hCD3e (B) mice. Mice were grouped when the tumor size was approximately 150±50mm3 (n=5). In the humanized mouse model, mPD-1 antibody significantly inhibited tumor growth, indicating normal T cell function. More aggressive tumor growth after anti-hCD3 antibody treatment was observed. This may result from activation induced cell death (AICD). As a result, the B-hCD3e mouse model is a powerful tool for in vivo CD3 antibody pharmacological efficacy studies.



