CDX Mouse Models & Anti-Tumor Efficacy
Biocytogen’s severely immune-deficient B-NDG mice are an ideal model for the engraftment of human peripheral blood mononuclear cells (PBMCs) and human CD34+ hematopoietic stem cells (hHSC). These human immune cell engraftment models are powerful tools for examining the effects of immuno-oncology agents on human immune cells.
Human Tumor Cell Models
Biocytogen has also engrafted multiple cell line-derived xenograft (CDX) models in B-NDG mice, including human solid tumor and hematological tumor cell lines. We have generated CDX mouse models from cancers such as lung carcinoma, colorectal adenocarcinoma, colon carcinoma, breast cancer, pancreatic carcinoma, bladder carcinoma, glioblastoma, liver cancer, and various leukemia and lymphoma cell lines. Additionally, we can develop custom CDX mouse models for subcutaneous, orthotopic, or metastatic tumor models based on our customer’s requirements. We provide pharmacodynamic (PD) services for any of these CDX mouse models.
-
Tumor Growth Kinetics of Selected CDX Models
-
Case 1: In vivo imaging of NOD-scid mice at 6, 16 and 28 days after inoculation with HT-29-GFP Luciferase Cells
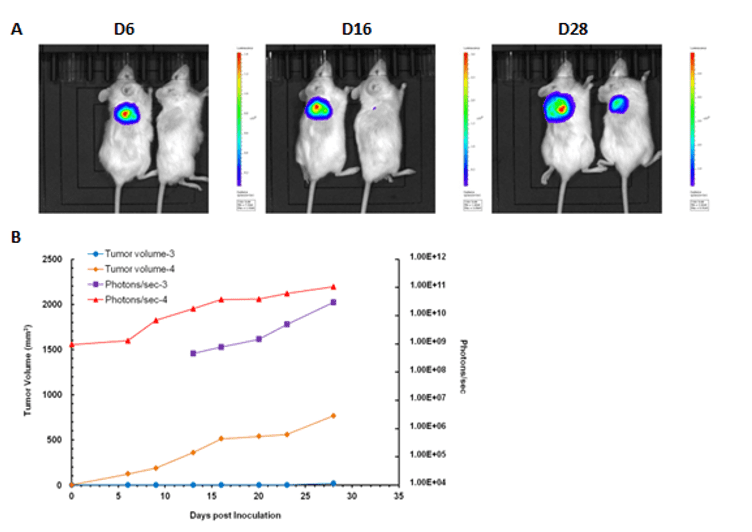
A) The mouse on the left was injected with 5 x 106 cells. The mouse on the right was injected with 5 x 105 (B) Signal intensity and tumor size graph.
Case 2: In vivo imaging of NOD-scid mice at 7, 14 and 21 days after inoculation with 4T1-Luciferase cells
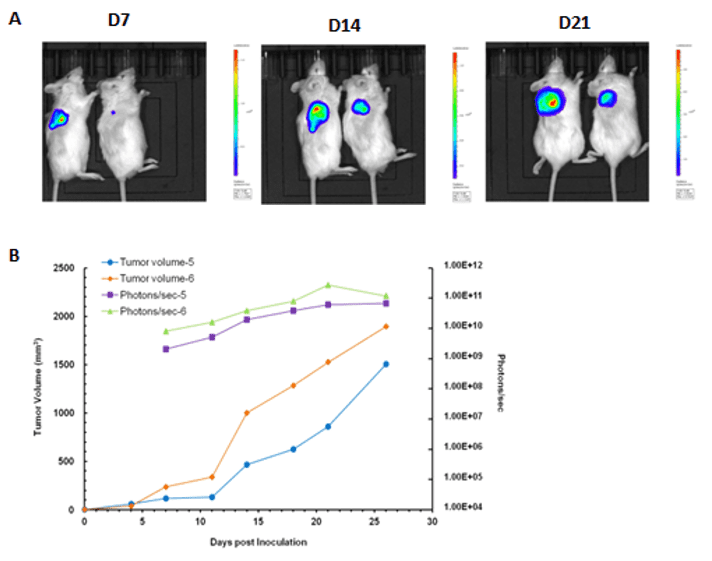
(A) The mouse on the left was injected with 5 x 106 cells; the mice on right was injected with 5 x 105 cells. (B) Signal intensity and tumor size graph.
Case 3: Human B cell lymphoma CDX model
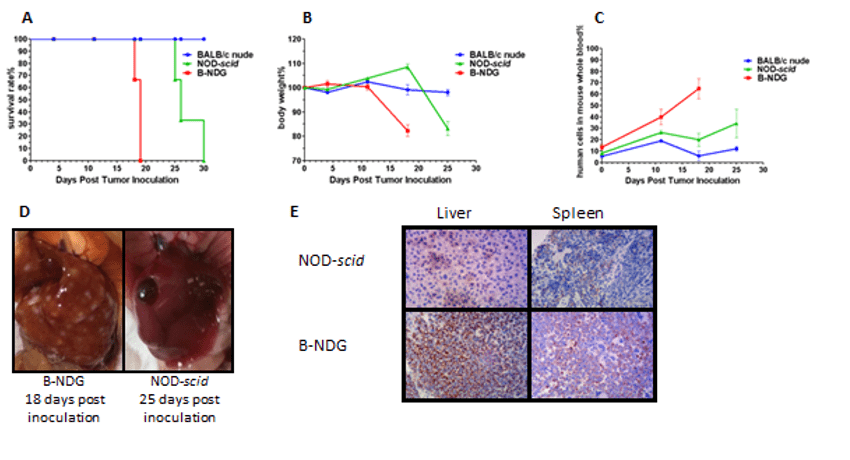 Raji cells (5×10⁶) were injected into B-NDG, NOD-scid and BALB/C nude mice. (A) Kaplan-Merier survival curves. (B) Relative body weight change. (C) Percentage of human cells in mouse peripheral blood quantified by q-PCR. (D) Liver tumor nodules. (E) Immunohistochemical staining of livers and spleens.
Raji cells (5×10⁶) were injected into B-NDG, NOD-scid and BALB/C nude mice. (A) Kaplan-Merier survival curves. (B) Relative body weight change. (C) Percentage of human cells in mouse peripheral blood quantified by q-PCR. (D) Liver tumor nodules. (E) Immunohistochemical staining of livers and spleens. -
Anti-CDX Tumor Efficacy Studies
-
Case 1
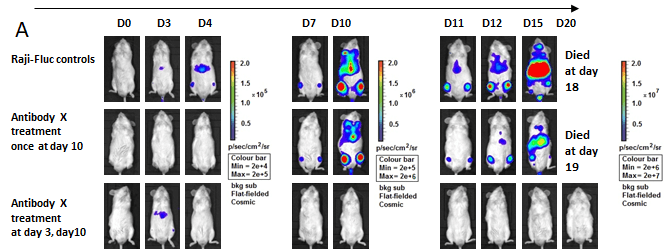
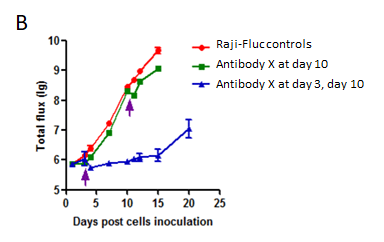
Raji-Fluc cells (5 x 10⁵) were injected into B-NDG mice. Antibody X was administered on specified days. (A) Representative in vivo imaging recorded at different time points to monitor tumor progression. (B) Tumor load (as indicated by luciferase activity) kinetic curves post antibody treatment. Early dosing on day 3 in addition to day 10 resulted in robust tumor growth inhibition. In contrast, a single dose at day 10 had little effect.
-
Anti-Tumor Efficacy in Human CD34+-Engrafted B-NDG Mice
-
Case 1
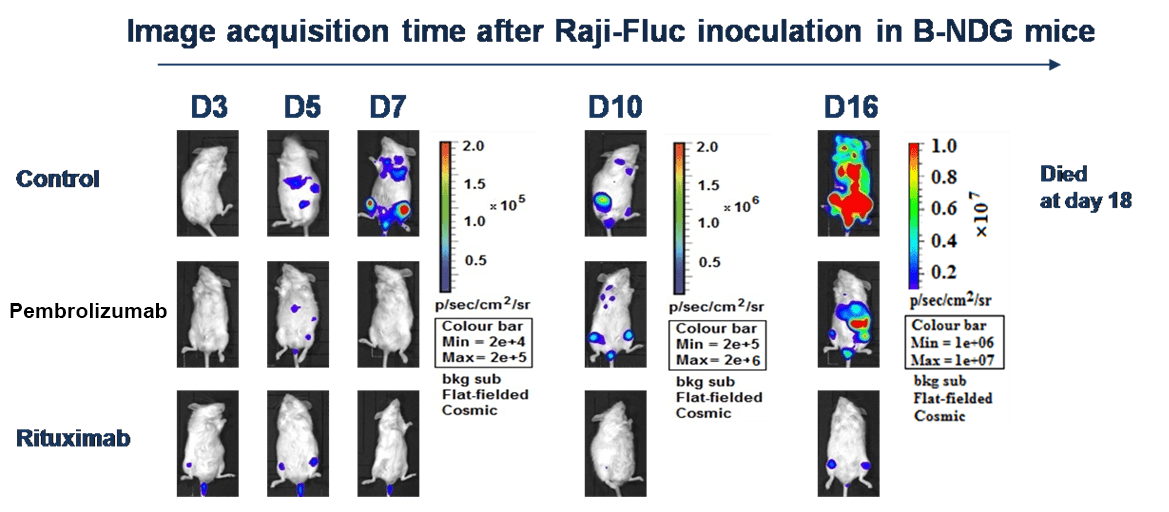
B-NDG mice engrafted with CD34+ HSC cells were used for in vivo efficacy assessment. Mice were treated with anti-human PD-1 antibody Pembrolizumab or anti-CD20 antibody Rituximab after Raji-Luc cell implantation. Dramatic tumor growth inhibition by both Pembrolizumab and Rituximab was observed.
Case 2
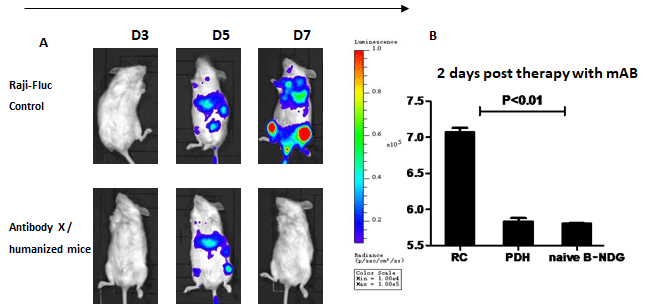
Humanized B-NDG mice engrafted with human CD34+ HSC were intravenously injected with 5 x 10⁵ Raji-Fluc cells. Five days after Raji-Fluc cells implantation, mice were treated with anti-human PD-1 antibody. (A) Images of tumor load as indicated by luciferase activity. Tumor growth was dramatically inhibited by the human PD-1 antibody at day 7. RC=Raji-Fluc + Control. PDH=Antibody X + Humanized Mice. Naive B-NDG=Untreated Group.
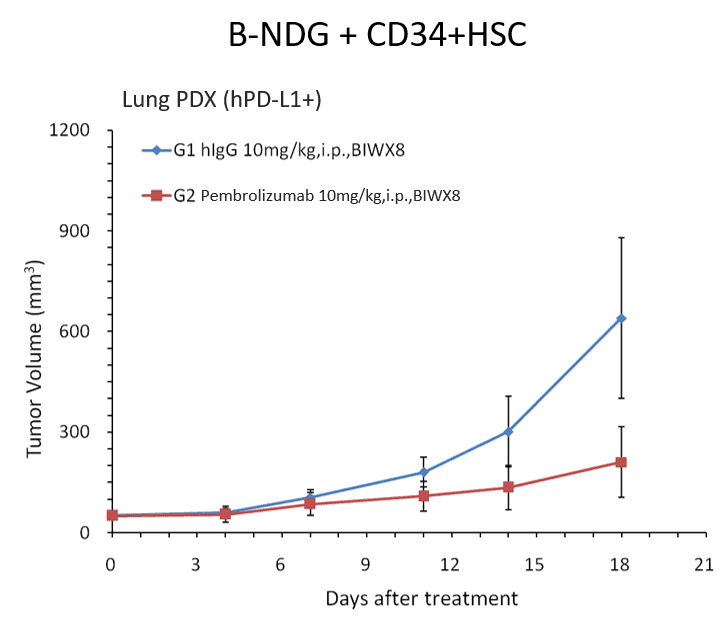
Efficacy evaluation of the human PD-1 antibody Pembrolizumab in CD34+ HSC engrafted B-NDG mice implanted with hPD-L1 positive lung PDX.
-
GvHD model in Human PBMC-Engrafted B-NDG Mice
-
Establishment of human PBMC engrafted GvHD model in B-NDG mice
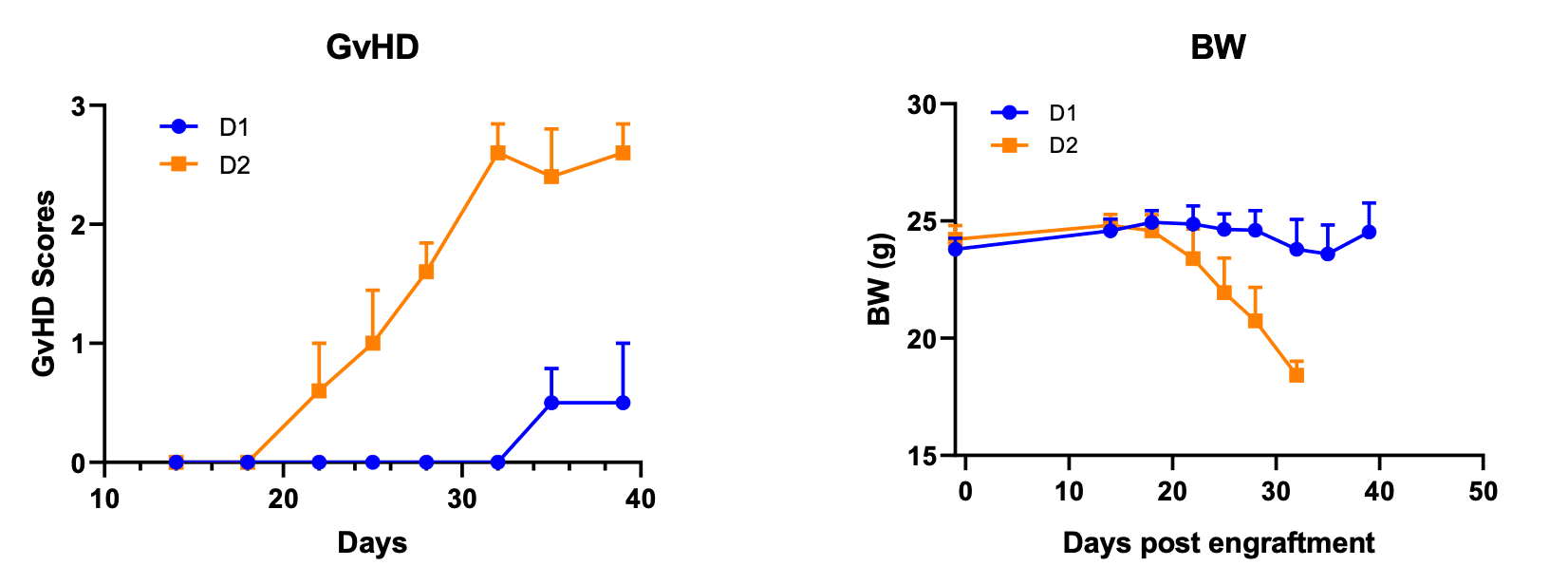
Figure 1. Establishment of human PBMC engrafted GvHD model in B-NDG mice.
B-NDG mice (female, 8-week old, n=5) were engrafted with 10 million human PBMC from two donors (D1, D2) on day 0. PBMC from donor 2 demonstrated more robust GvHD than those from donor 1. GvHD were scored according to Table 1.
Checkpoint inhibitors aggravated GvHD in tumor-bearing B-NDG mice
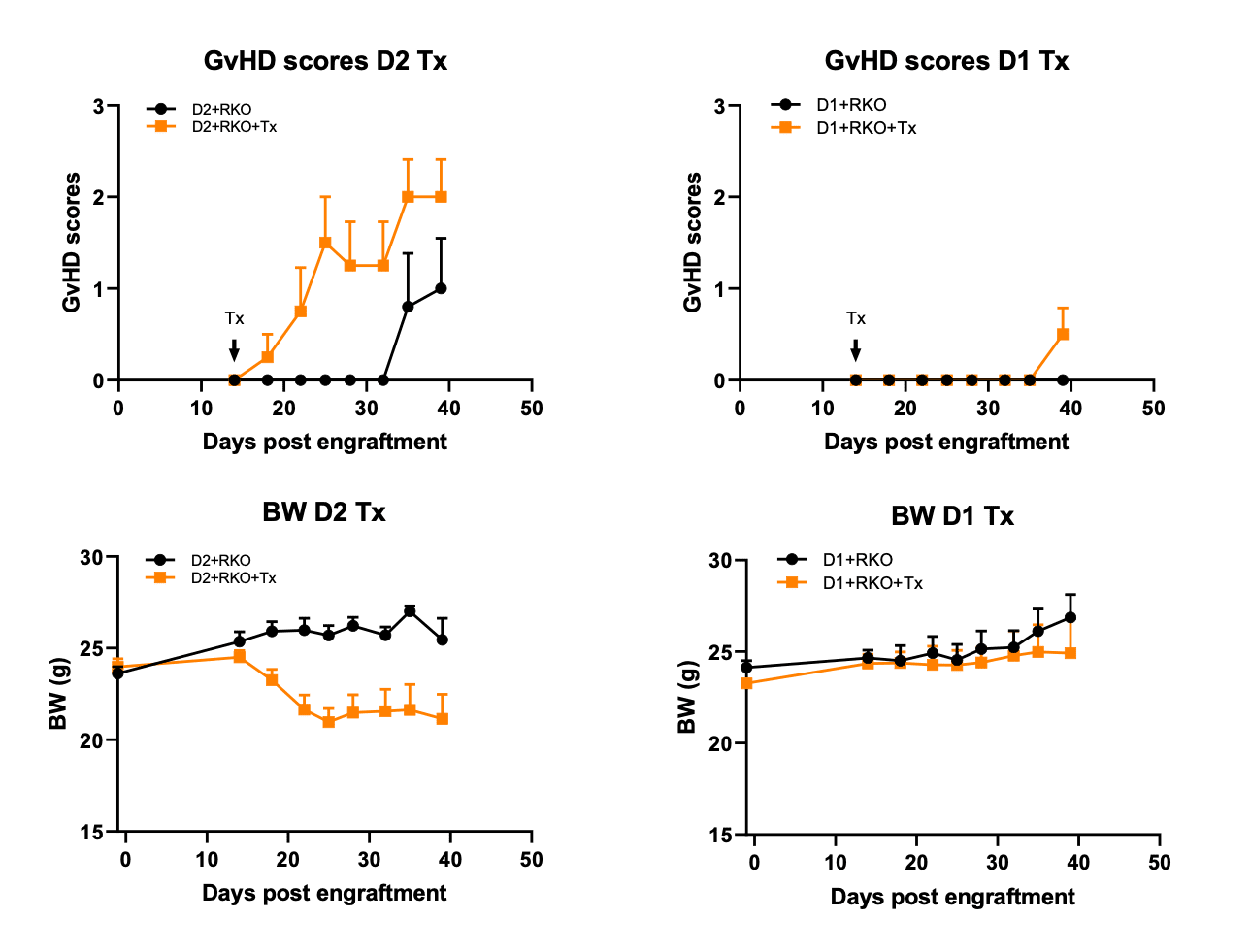
Figure 2. Checkpoint inhibitors aggravated GvHD in RKO tumor-bearing B-NDG mice.
B-NDG mice (female, 8-week old, n=5) bearing RKO tumor (5 million, 50% Matrigel, inoculated subcutaneously on day 4) were engrafted with 10 million human PBMC from two donors (D1, D2) on day 0. Treatment by 125 ug per mouse pembrolizumab and ipilimumab, BIW, i.p. started on day 14. Checkpoint inhibition resulted in earlier onset and more severe GvHD, with more prominent effects with PBMC from donor 2, which had more robust GvHD than PBMC from donor 1. GvHD scores are shown in top panels and body weight (BW) changes in low panels. GvHD was scored according to Table 1.
Pharmacological validation of GvHD model in B-NDG mice: Reduction of GvHD by corticosteroid drug
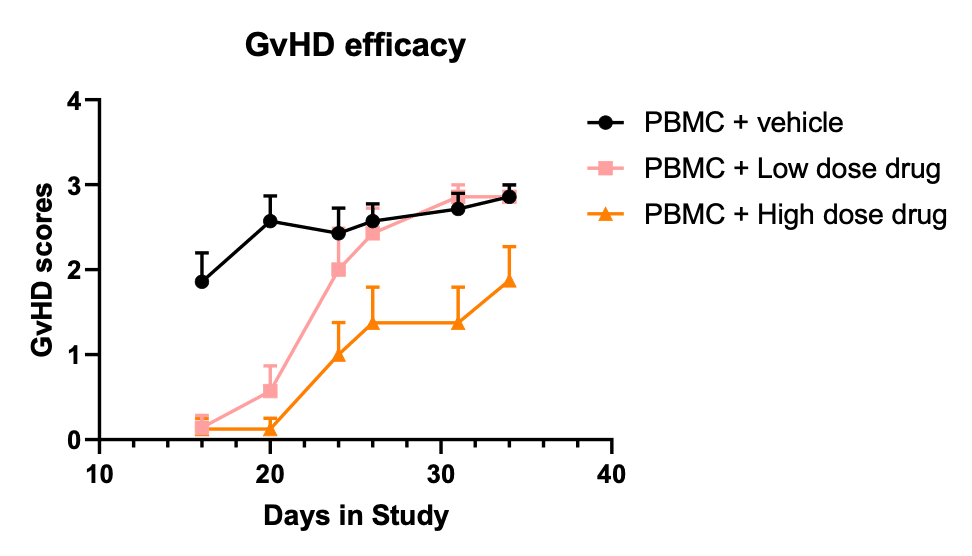
Figure 3. Pharmacological validation of GvHD model in B-NDG mice: alleviation of GvHD by corticosteroid drug.
B-NDG mice (female, 8-week old, n=7-8) were engrafted with 20 million human PBMC (iv) on day 0. Drug treatment (i.m., qdx4) started on day 7. Robust GvHD developed in the absence of treatment. Corticosteroid drug dose-dependently controlled the disease. High dose (1 mg/kg) of the drug GvHD resulted in delayed onset and reduced severity of symptoms while low dose (0.1 mg/kg) of the drug led to intermediated effect. GvHD was scored according to Table 1.
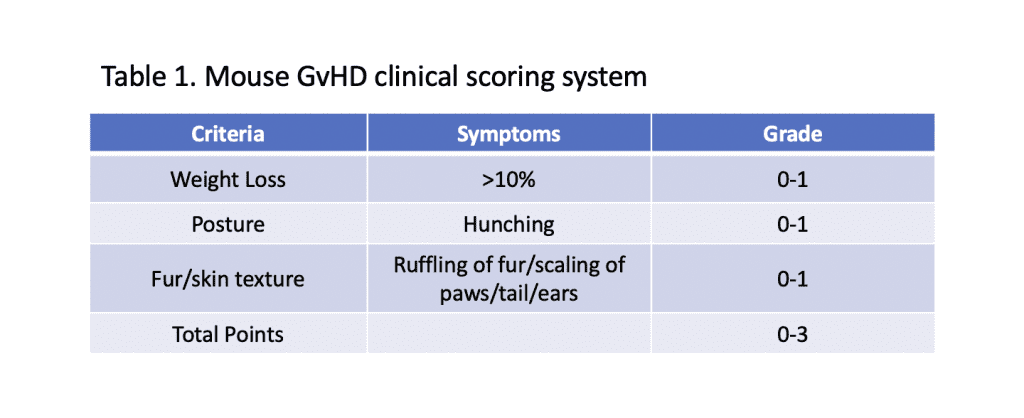
-
Anti-Tumor Efficacy in Human PBMC-Engrafted B-NDG Mice
-
Checkpoint inhibitors showed encouraging anti-tumor activity in PBMC engrafted RKO/B-NDG model
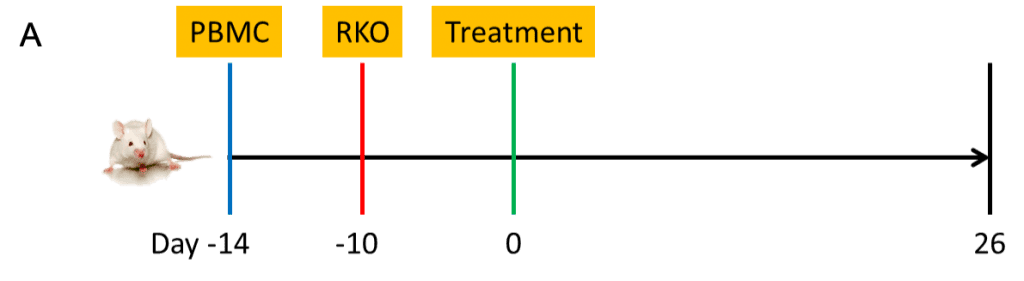
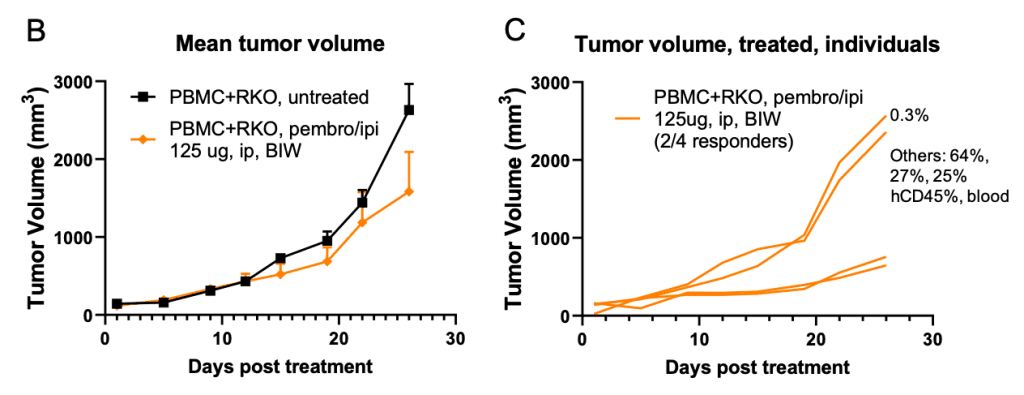
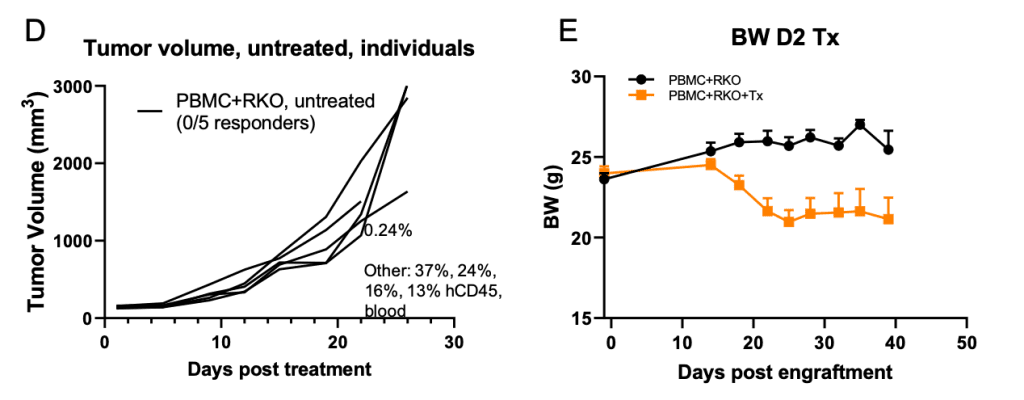
Figure 1. Checkpoint inhibitors showed encouraging anti-tumor activity in PBMC engrafted RKO/B-NDG model.
B-NDG mice (female, 8-week old, n=4-5) were engrafted with 10 million human PBMC (iv) and 5 M RKO cells (in 50% Matrigel, inoculated subcutaneously) according to Panel A. Tumor volume (B-D) and body weight (E) were monitored over time. Percent of human CD45 cells in total human and mouse CD45 cells in terminal bleeds were indicated (C, D). Data were expressed as mean +/- SEM. TGI: tumor growth inhibition. Pembro/ipi: pembrolizumab/ipilimumab.
Checkpoint inhibitors increased CD8 content and CD8/Treg ratio significantly in blood in PBMC RKO/B-NDG model; Dichotomy of responders vs non-responders observed in tumor & blood
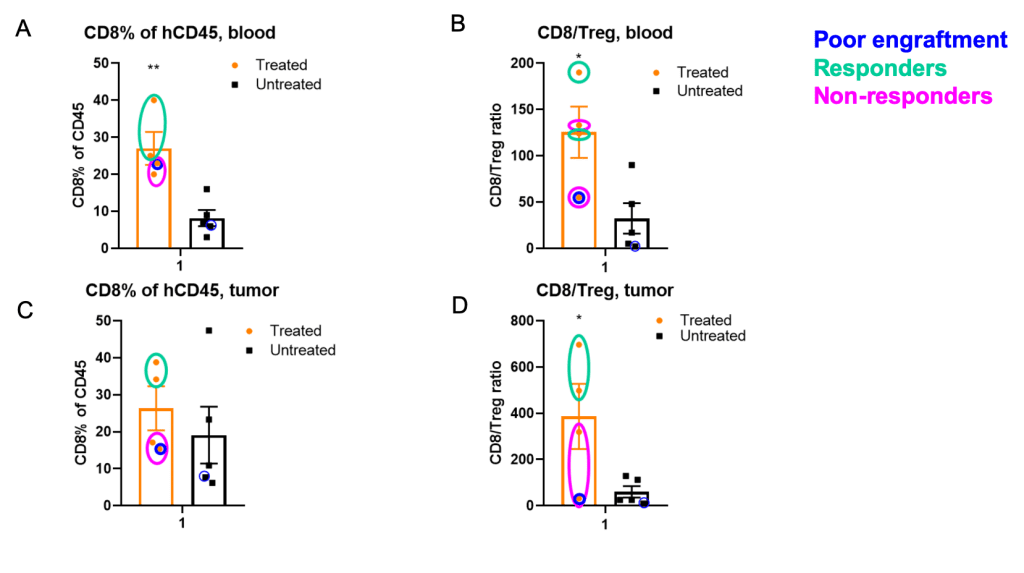 Figure 2. Checkpoint inhibitors increased CD8 content and CD8/Treg ratio significantly in blood in PBMC RKO/B-NDG model.
Figure 2. Checkpoint inhibitors increased CD8 content and CD8/Treg ratio significantly in blood in PBMC RKO/B-NDG model.Flow cytometry analysis of terminal blood (A,B) and tumor (C, D) samples were performed. A dichotomy of responders vs non-responders to checkpoint inhibition was observed in tumor and blood. Blue circles: poor (<1%) human PBMC engraftment; green and magenta circles: responders and non-responders in Figure 2C, respectively.
GvHD of RKO-bearing B-NDG mice
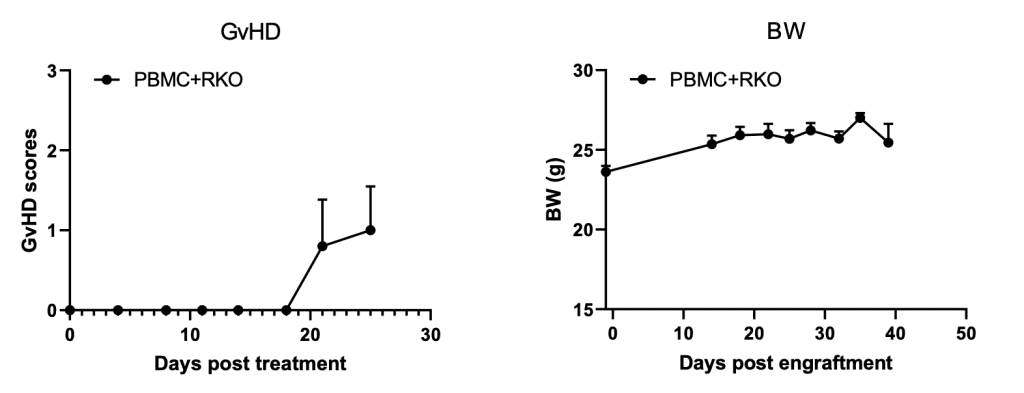
Figure 3. GvHD and body weight (BW) of RKO-bearing B-NDG mice. GvHD were scored according to Table 1.
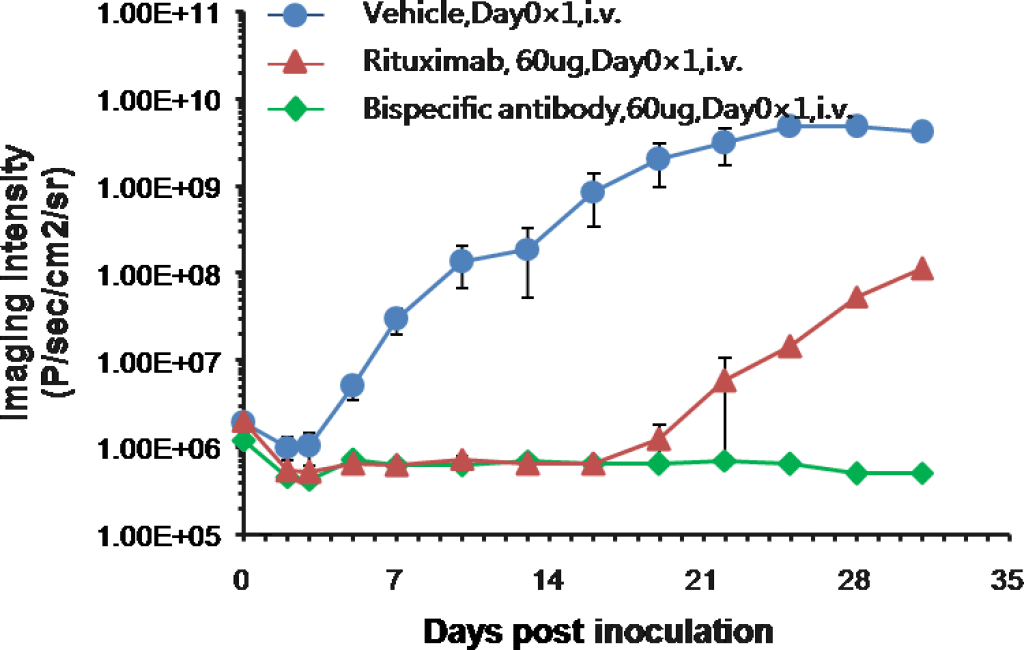
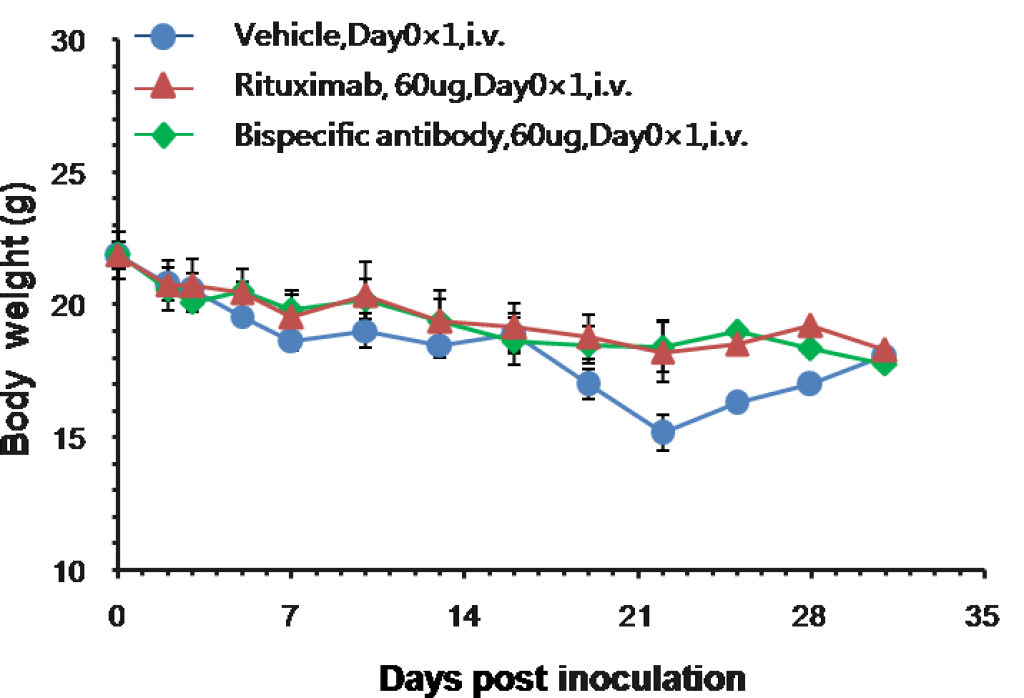
Efficacy study of monoclonal and bispecific antibodies in B-NDG mice engrafted with human PBMC and Raji-Luc B lymphoma.



