Obesity is a chronic disease that results from an imbalance of endogenous and environmental exposures, such as basal metabolic rate, energy expenditure, and food intake. Of note, excessive caloric intake and energy-dense meals are the leading causes of obesity. Surrogate animal models have been developed to study obesity-related complications. At Biocytogen, we have developed a high-fat diet-induced mouse model, which consists of 60 kcal%, to study pathophysiological changes associated with diabetes.
-
High-Fat Diet-Induced Obese (DIO) Mouse Model
-
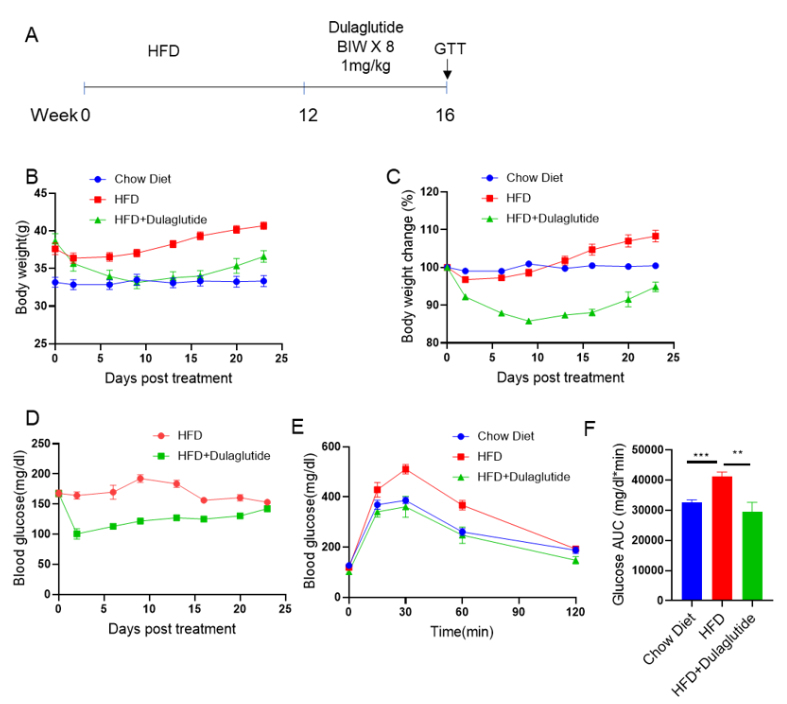
Anti-obesity effect of Dulalgutide in a high-fat diet-induced obese (DIO) mouse model. (A) C57BL/6 mice were fed a high-fat diet (60 kcal%) for 12 weeks to induce obesity. Dulaglutide (in house) was subcutaneously injected twice per week for 4 weeks after grouping. (B,C) Body weight, (D,E) blood glucose, and (F) glucose tolerance tests were recorded (n=5 mice per group). Values are expressed as mean ± SEM. *** p<0.001, ** p<0.01.
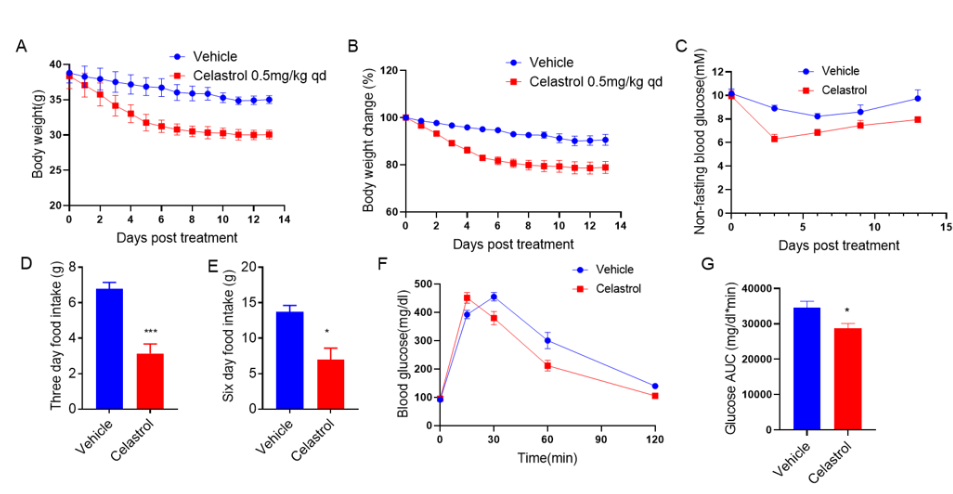
Anti-obesity effect of Celastrol in a high-fat diet-induced obese (DIO) mouse model. C57BL/6 mice were fed a high-fat diet (60 kcal%) for 12 weeks to induce obesity. Mice were injected with either vehicle control or Celastrol (0.5mg/kg) intraperitoneally once daily for 14 days. (A, B) Body weight, (C) blood glucose, (D, E) food intake and (F, G) glucose tolerance test results were measured. Celastrol treatment controlled obesity-related levels. Values are expressed as mean ± SEM. *** p<0.001. (n=5 mice per group).
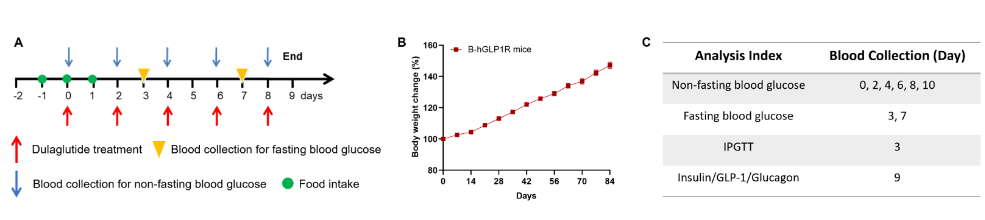
Model schematic and in vivo efficacy of Dulaglutide in humanized B-hGLP1R mice. B-hGLP1R mice (male, n=10) were fed a high-fat diet for 12 weeks to induce obesity. The mice were grouped randomly according to body weight. After that, Dulaglutide (in house) was subcutaneously injected every two days from Day 0 to 8. Collect blood and measure blood glucose and IPGTT on the days shown in the schematic diagram and the table. Meanwhile, measure plasma insulin during the IPGTT.
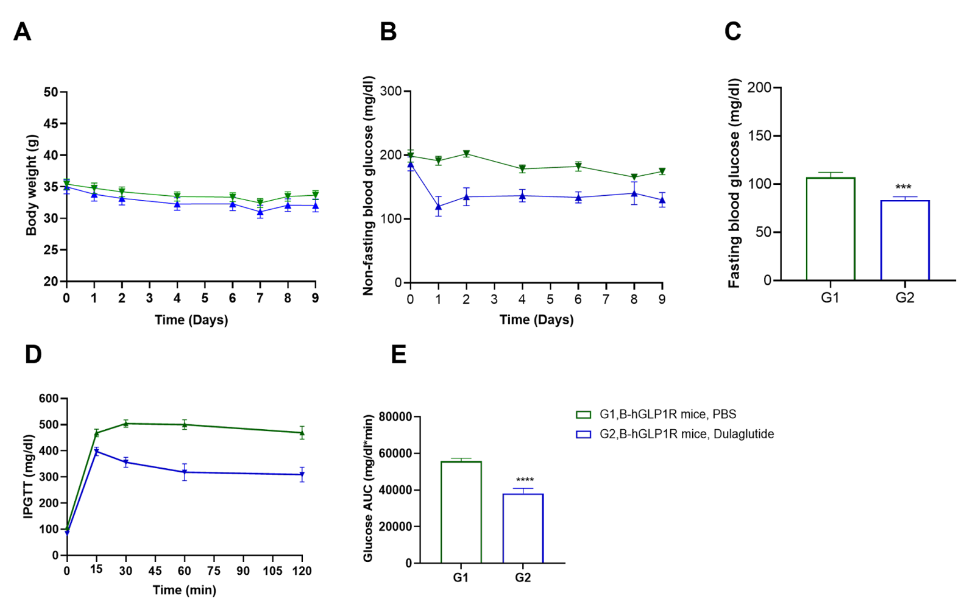
Dulaglutide treatment reduces blood glucose in humanized B-hGLP1R obese mice. Blood was collected from B-hGLP1R mice (male, n=10) at multiple time points following injection of Dulaglutide (in house) or PBS. (A) Body weight, (B) non-fasting blood glucose, (C) fasting blood glucose, (D) serum glucose levels after intraperitoneal glucose challenge, and (E) area under the curve were measured in B-hGLP1R obese mice treated with either Dulaglutide or PBS. Without negatively impacting body weight changes (A), Dulaglutide treatment reduced non-fasting (B) and fasting (C) blood glucose levels compared to PBS control. Furthermore, glucose tolerance via IPGTT was improved (D,E) in Dulaglutide-treated B-hGLP1R obese mice.
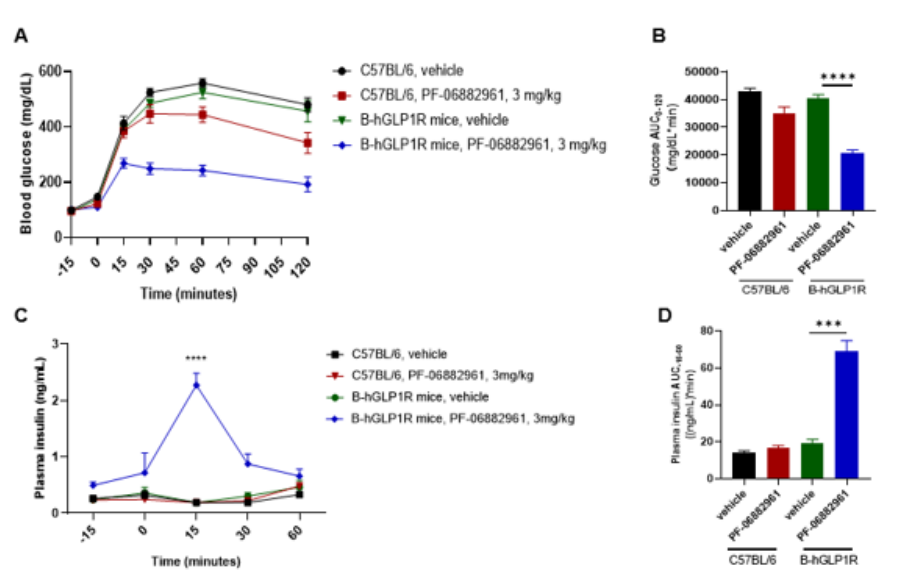
PF-06882961 improved glucose tolerance and increased insulin in B-hGLP1R mice. IPGTT study in C57BL/6 (n=6) and B-hGLP1R mice (n=6) after subcutaneous dosing with vehicle or PF-06882961 3 mg/kg. (A) Blood glucose; (B) Blood glucose AUC0-120 ; (C) Plasma insulin; (D) Plasma insulin AUC-15-60; Glucose tolerance was improved by PF-06882961 in B-hGLP1R mice but not in wild-type C57BL/6 mice, and increase more insulin in plasma. Values are expressed as mean ± SEM.



