Basic Information
-
Targeting strategy

-
Gene targeting strategy for B-hTLR8/hTLR7 mice. The exon 3 of mouse Tlr8 gene that encodes the extracellular domain was replaced by human TLR8 exon 3 B-hTLR8/hTLR7 mice. The exon 3 of mouse Tlr7 gene that encodes the extracellular domain was replaced by human TLR7 exon 3 in B-hTLR8/hTLR7 mice.
-
mRNA expression analysis

-
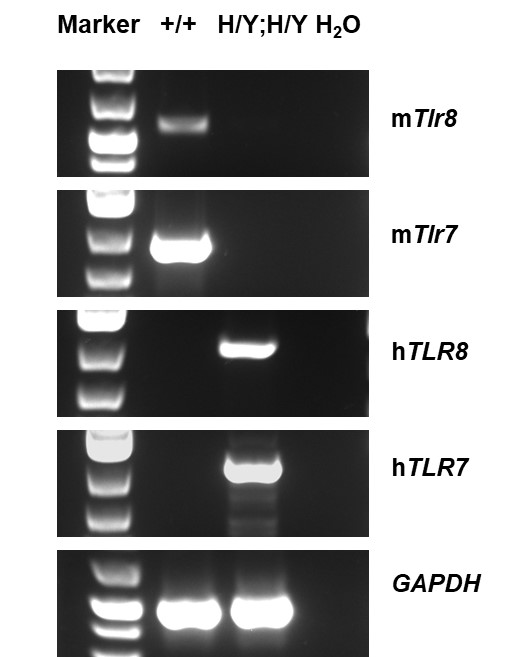
Strain specific analysis of TLR8 and TLR7 gene expression in wild-type C57BL/6 mice and homozygous B-hTLR8/hTLR7 mice by RT-PCR. Mouse Tlr8 and Tlr7 mRNA was detectable in wild-type C57BL/6 mice (+/+). Human TLR8 and TLR7 mRNA was detectable only in homozygous B-hTLR8/hTLR7 mice but not in wild-type mice.
-
Protein expression analysis in monocytes

-
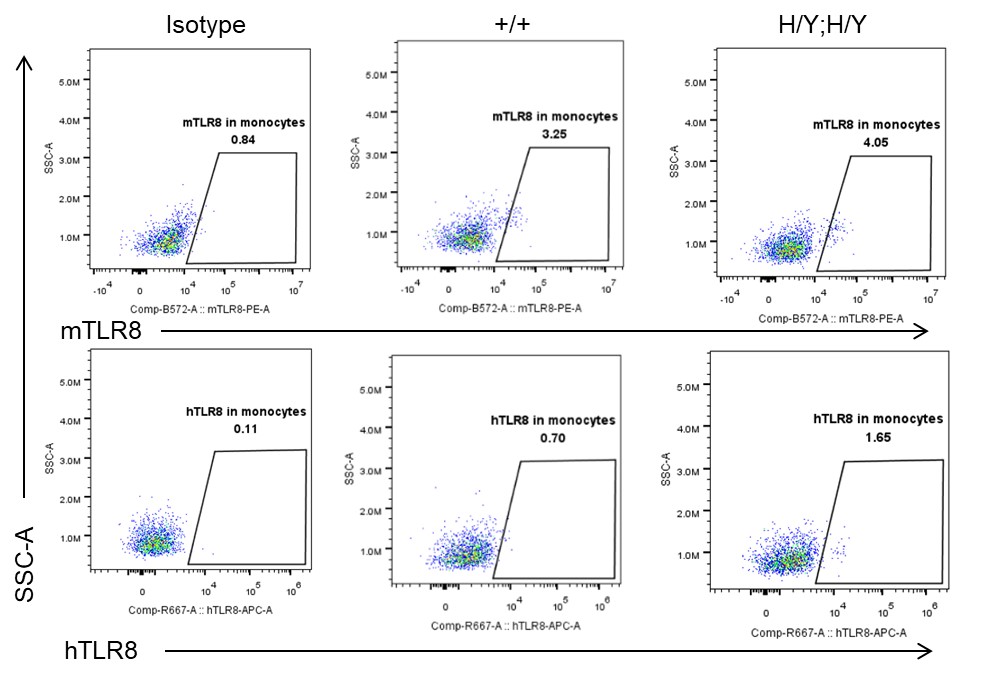
Strain specific TLR8 expression analysis in homozygous B-hTLR8/hTLR7 mice by flow cytometry. Splenocytes were collected from wild-type mice (+/+) and homozygous B-hTLR8/hTLR7 mice (H/Y;H/Y), and analyzed by flow cytometry with species-specific anti-TLR8 antibody. Mouse TLR8 was detectable in wild-type mice and homozygous B-hTLR8/hTLR7 mice due to the anti-mouse TLR8 antibody cross-reacts with human TLR8. Human TLR8 was exclusively detectable in homozygous B-hTLR8/hTLR7 mice but not WT mice.
-
Protein expression analysis in macrophages

-
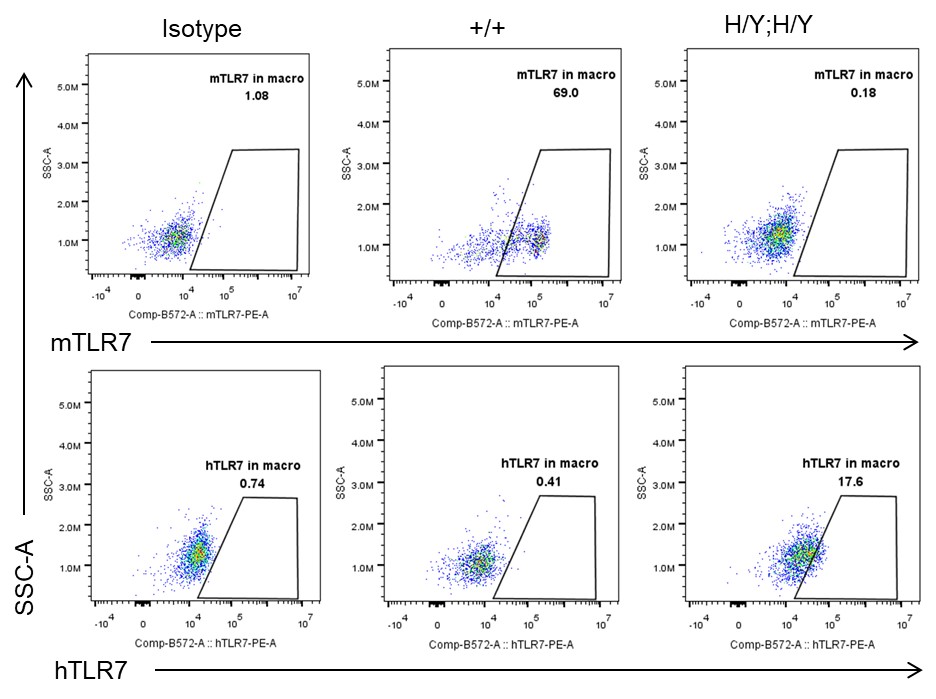
Strain specific TLR7 expression analysis in homozygous B-hTLR8/hTLR7 mice by flow cytometry. Splenocytes were collected from wild-type mice (+/+) and homozygous B-hTLR8/hTLR7 mice (H/Y;H/Y), and analyzed by flow cytometry with species-specific anti-TLR7 antibody. Mouse TLR7 was exclusively detectable in WT mice but not in homozygous B-hTLR8/hTLR7 mice. Human TLR7 was exclusively detectable in homozygous B-hTLR8/hTLR7 mice but not WT mice.
-
Protein expression analysis in DCs

-
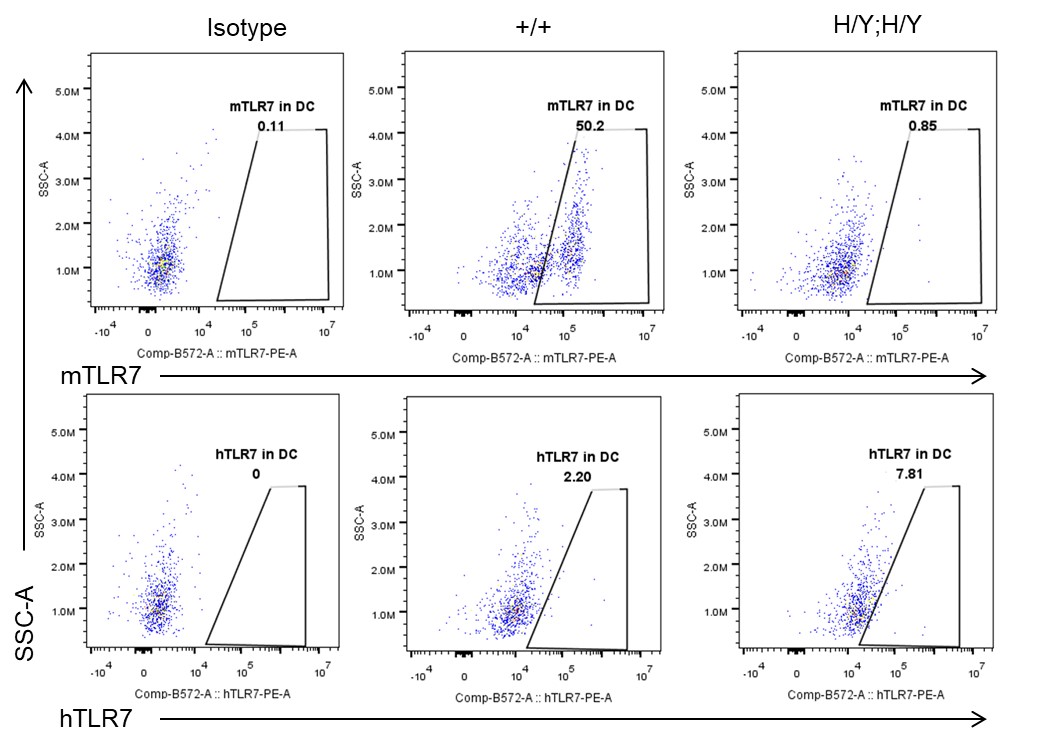
Strain specific TLR7 expression analysis in homozygous B-hTLR8/hTLR7 mice by flow cytometry. Splenocytes were collected from wild-type mice (+/+) and homozygous B-hTLR8/hTLR7 mice (H/Y;H/Y), and analyzed by flow cytometry with species-specific anti-TLR7 antibody. Mouse TLR7 was exclusively detectable in WT mice but not in homozygous B-hTLR8/hTLR7 mice. Human TLR7 was exclusively detectable in homozygous B-hTLR8/hTLR7 mice but not WT mice.
-
Analysis of leukocytes cell subpopulation in spleen

-
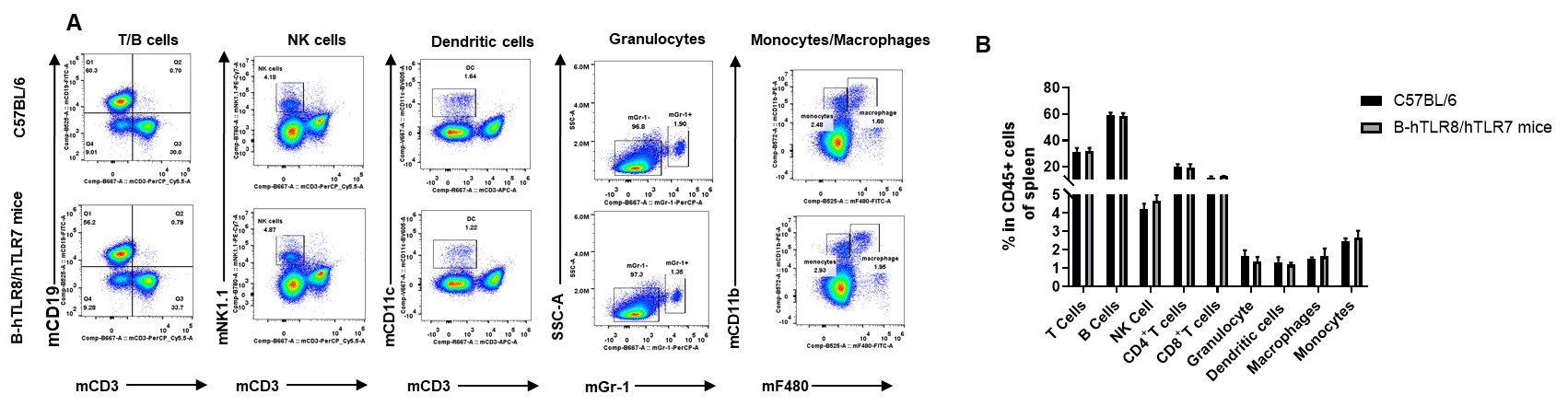
Analysis of spleen leukocyte subpopulations by FACS. Splenocytes were isolated from female C57BL/6 and B-hTLR8/hTLR7 mice (n=3, 8-week-old). Flow cytometry analysis of the splenocytes was performed to assess leukocyte subpopulations. A. Representative FACS plots. Single live cells were gated for the CD45+ population and used for further analysis as indicated here. B. Results of FACS analysis. Percent of T cells, B cells, NK cells, dendritic cells, granulocytes, monocytes and macrophages in homozygous B-hTLR8/hTLR7 mice were similar to those in the C57BL/6 mice, demonstrating that TLR8 humanized does not change the overall development, differentiation or distribution of these cell types in spleen.
-
Analysis of T cell subpopulation in spleen

-
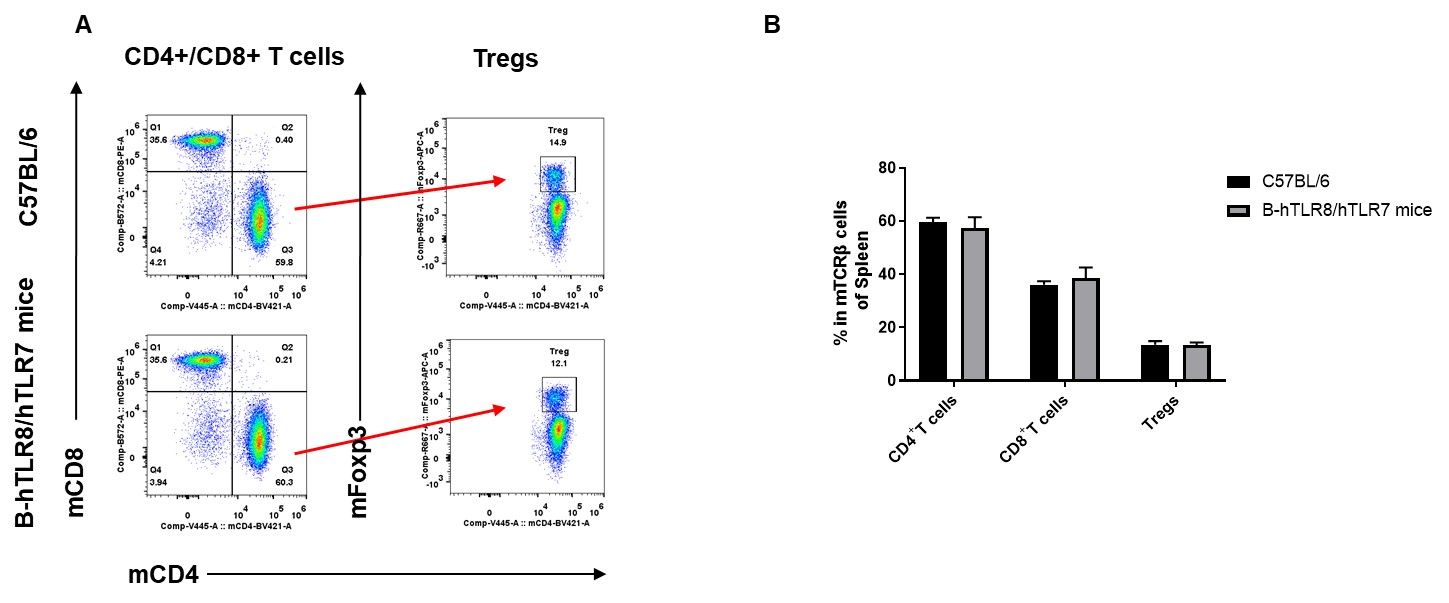
Analysis of spleen T cell subpopulations by FACS. Splenocytes were isolated from female C57BL/6 and B-hTLR8/hTLR7 mice (n=3, 8-week-old). Flow cytometry analysis of the splenocytes was performed to assess leukocyte subpopulations. A. Representative FACS plots. Single live CD45+ cells were gated for TCRβ+ T cell population and used for further analysis as indicated here. B. Results of FACS analysis. The percent of CD8+ T cells , CD4+ T cells and Tregs in homozygous B-hTLR8/hTLR7 mice were similar to those in the C57BL/6 mice, demonstrating that TLR8 humanized does not change the overall development, differentiation or distribution of these cell types in spleen.
-
Analysis of leukocytes cell subpopulation in blood

-
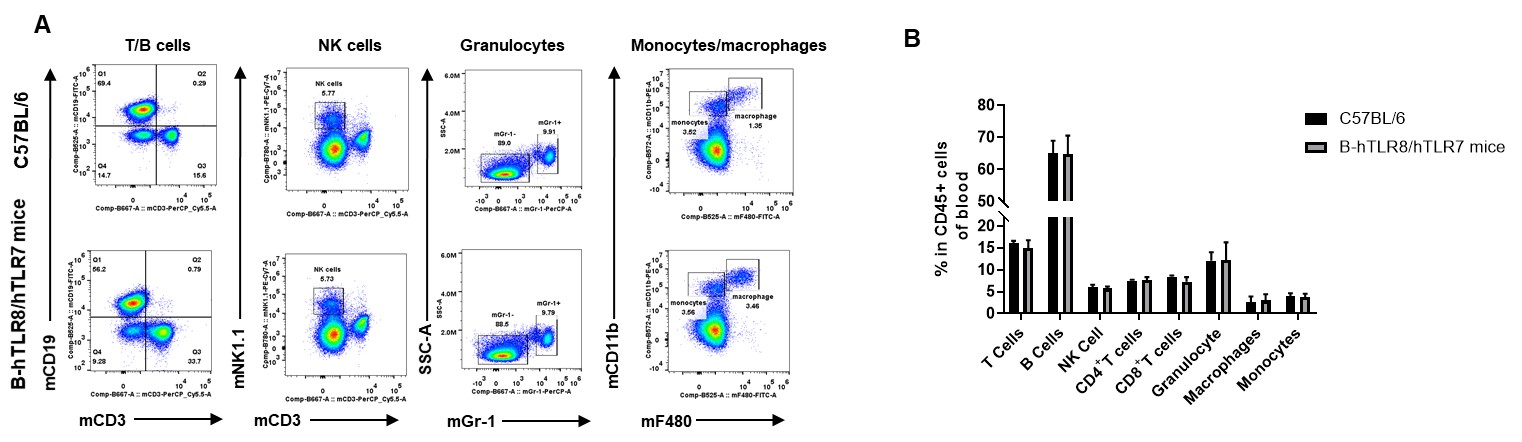
Analysis of blood leukocyte subpopulations by FACS. Blood were isolated from female C57BL/6 and B-hTLR8/hTLR7 mice (n=3, 8-week-old). Flow cytometry analysis of the blood was performed to assess leukocyte subpopulations. A. Representative FACS plots. Single live cells were gated for the CD45+ population and used for further analysis as indicated here. B. Results of FACS analysis. Percent of T cells, NK cells, dendritic cells, granulocytes, monocytes and macrophages in homozygous B-hTLR8/hTLR7 mice were similar to those in the C57BL/6 mice, demonstrating that TLR8 humanized does not change the overall development, differentiation or distribution of these cell types in blood.
-
Analysis of T cell subpopulation in blood

-
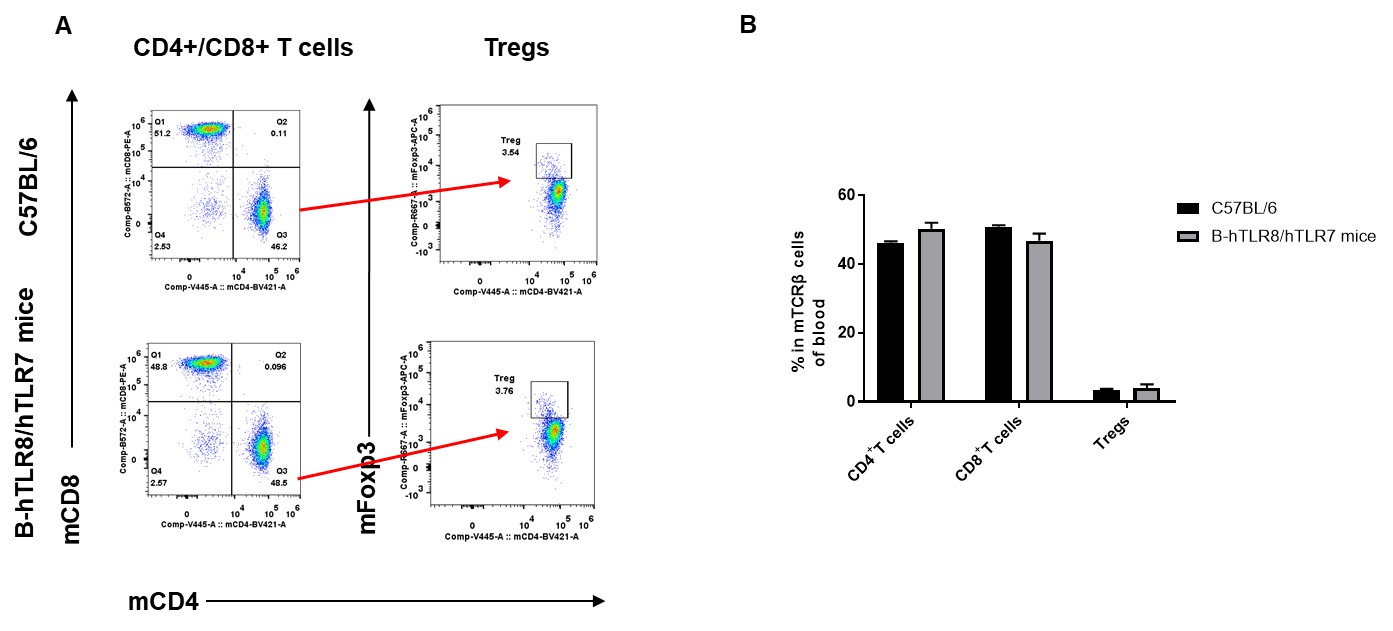
Analysis of blood T cell subpopulations by FACS. Blood were isolated from female C57BL/6 and B-hTLR8/hTLR7 mice (n=3, 8-week-old). Flow cytometry analysis of the blood was performed to assess leukocyte subpopulations. A. Representative FACS plots. Single live CD45+ cells were gated for CD3+ T cell population and used for further analysis as indicated here. B. Results of FACS analysis. The percent of CD4+ T cells, CD8+ T cells, and Tregs in homozygous B-hTLR8/hTLR7 mice were similar to those in the C57BL/6 mice, demonstrating that introduction of hTLR8 in place of its mouse counterpart does not change the overall development, differentiation or distribution of these T cell subtypes in blood.
-
Analysis of leukocytes cell subpopulation in lymph node

-
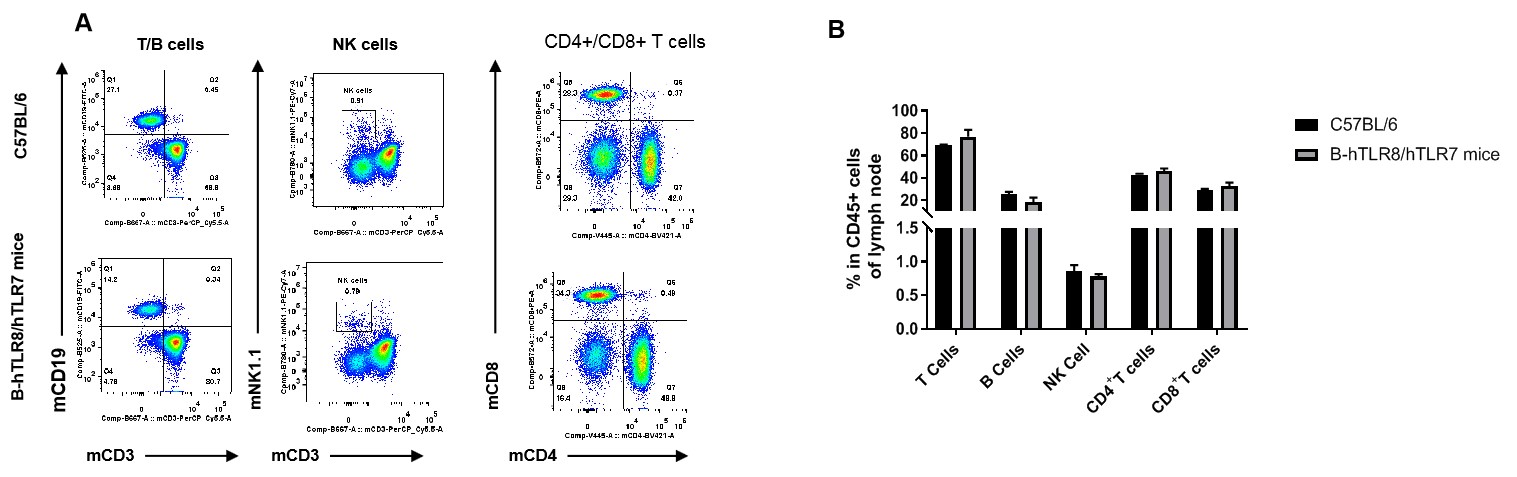
Analysis of lymph node leukocyte subpopulations by FACS. Lymph node cells were isolated from female C57BL/6 and B-hTLR8/hTLR7 mice (n=3, 8-week-old). Flow cytometry analysis of the cells was performed to assess leukocyte subpopulations. A. Representative FACS plots. Single live cells were gated for the CD45+ population and used for further analysis as indicated here. B. Results of FACS analysis. Percent of T cells, B cells, NK cells,CD4+ T cells,CD8+ T cells in homozygous B-hTLR8/hTLR7 mice were similar to those in the C57BL/6 mice, demonstrating that TLR8 humanized does not change the overall development, differentiation or distribution of these cell types in lymph node.
-
Analysis of T cell subpopulation in lymph node

-
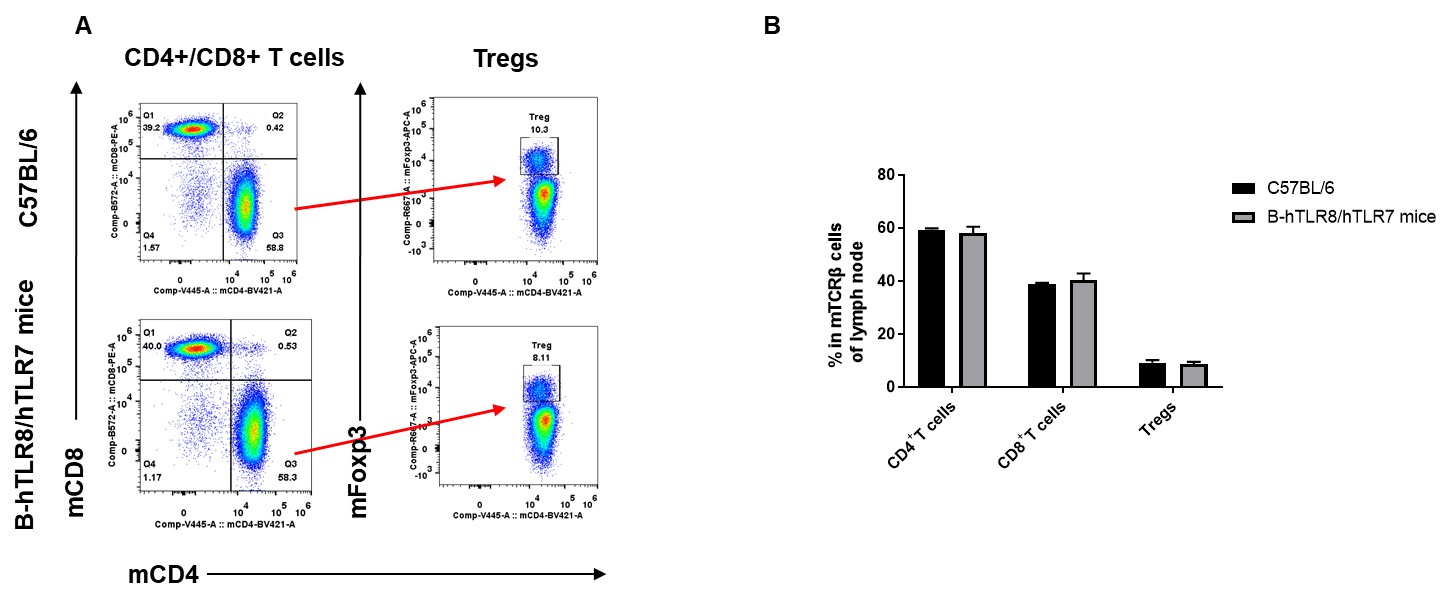
Analysis of lymph node T cell subpopulations by FACS. lymph node cells were isolated from female C57BL/6 and B-hTLR8/hTLR7 mice (n=3, 8-week-old). Flow cytometry analysis of the cells was performed to assess leukocyte subpopulations. A. Representative FACS plots. Single live CD45+ cells were gated for TCRβ+ T cell population and used for further analysis as indicated here. B. Results of FACS analysis. The percent of Tregs in homozygous B-hTLR8/hTLR7 mice were similar to those in the C57BL/6 mice, demonstrating that TLR8 humanized does not change the overall development, differentiation or distribution of these cell types in lymph node.
-
Analysis of mTNF-α and mIFN-γ protein expression by ELISA

-
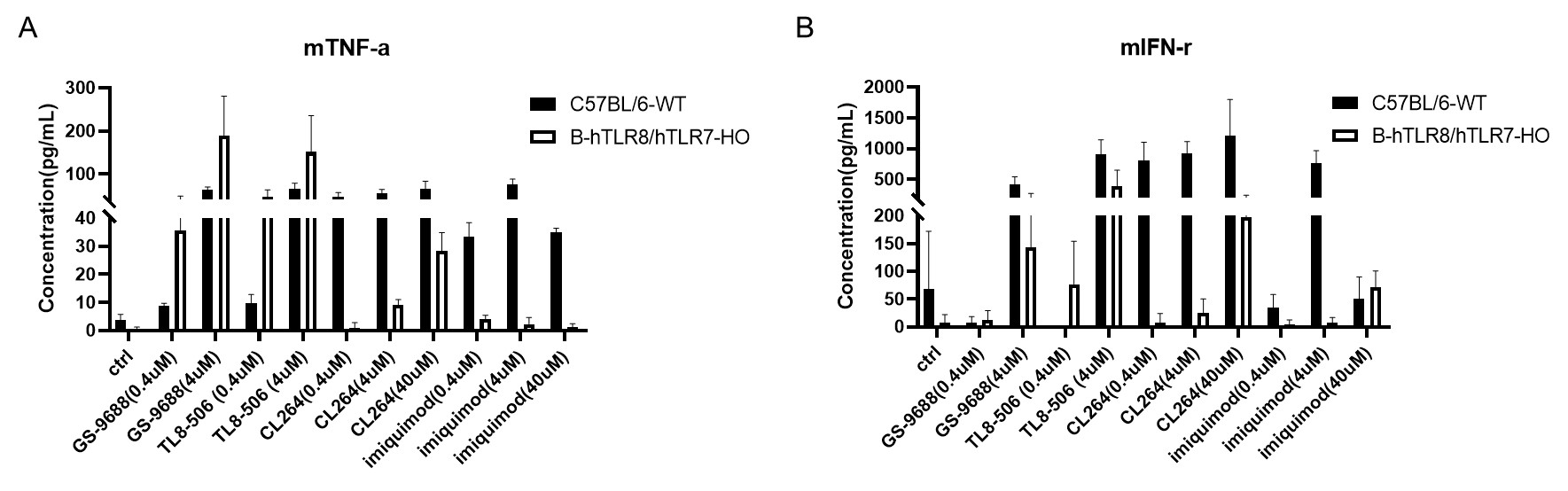
Strain specific mTNF-α and mIFN-γ expression analysis in homozygous B-hTLR8/hTLR7 mice by ELISA. Splenocytes were isolated from wild-type C57BL/6 mice (+/+) and homozygous B-hTLR8/hTLR7(H/Y, H/Y) and stimulated with TLR8/TLR7 agonist in vitro for 24 h, then cell supernatants were collected and analyzed by ELISA with species-specific mTNF-α (A) and mIFN-γ (B) ELISA kit.
-
Summary

-
mRNA expression analysis:
Mouse Tlr8 and Tlr7 mRNA was detectable in wild-type C57BL/6 mice (+/+). Human TLR8 andTLR7 mRNA was detectable only in homozygous B-hTLR8/hTLR7 mice but not in wild-type mice.
Protein expression analysis:
- Mouse TLR8 was detectable in WT mice and homozygous B-hTLR8/hTLR7 mice due to the anti-mouse TLR8 antibody cross-reacts with human TLR8. Human TLR8 was exclusively detectable in homozygous B-hTLR8/hTLR7 mice but not WT mice.
- Mouse TLR7 was exclusively detectable in WT mice but not in homozygous B-hTLR8/hTLR7 mice. Human TLR7 was exclusively detectable in homozygous B-hTLR8/hTLR7 mice but not WT mice.
Leukocytes cell subpopulation analysis:
TLR8 and TLR7 humanized does not change the overall development, differentiation or distribution of immune cell types in spleen, blood and lymph node in B-hTLR8/hTLR7 mice.
Functional analysis:
TLR8 agonists GS-9688 or TL8-506 and TLR7 agonist CL264 can activate mTNF-α and mIFN-γ in both wild-type C57BL/6 mice (+/+) and homozygous B-hTLR8/hTLR7 mice.


