Basic Information
-
mRNA expression analysis

-
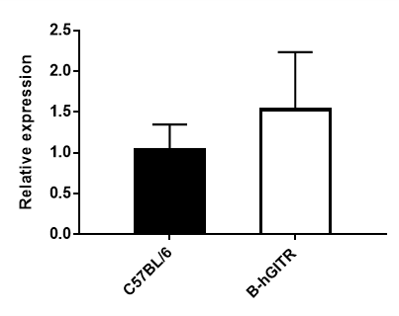
qPCR analysis of the GITR gene. The GITR mRNA isolated from spleen was detected by qPCR analysis. No significant difference was observed between C57BL/6 and B-hGITR mice (n=3).
-
Protein expression analysis

-

Strain specific GITR expression analysis in homozygous B-hGITR mice by flow cytometry. Splenocytes were collected from WT and homozygous B-hGITR (H/H) mice stimulated with anti-CD3ε in vivo (7.5 μg/mice), and analyzed by flow cytometry with species-specific anti-GITR antibody. Mouse GITR was exclusively detected in WT. Human GITR was exclusively detected in homozygous B-hGITR but not WT mice.
-
Analysis of leukocytes cell subpopulation in B-hGITR mice

-
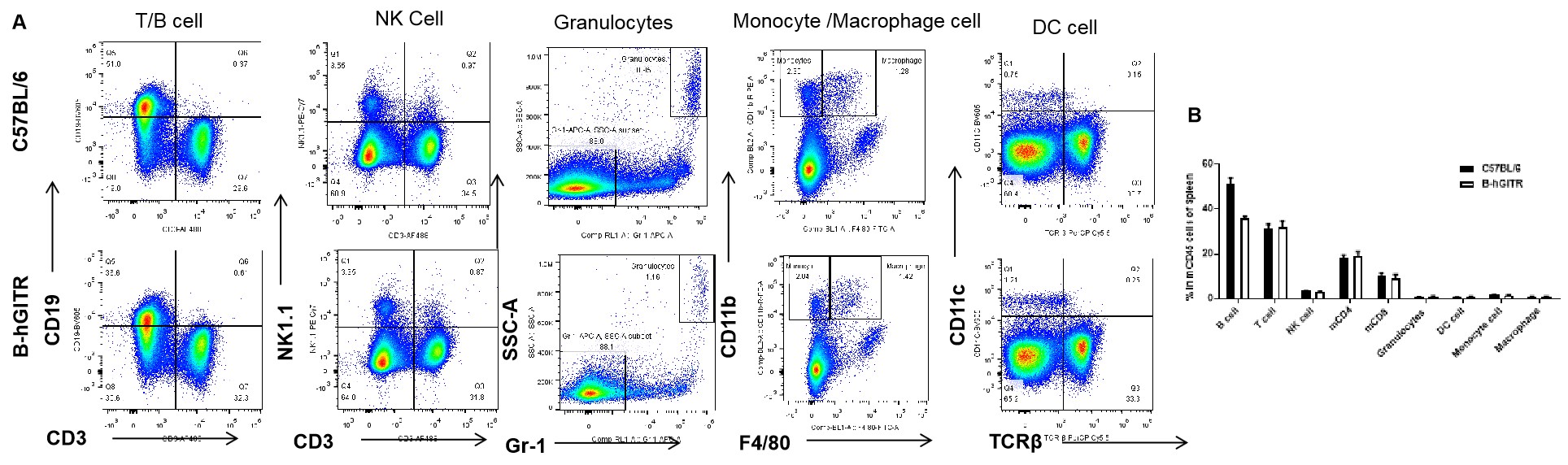
Analysis of spleen leukocyte subpopulations by FACS. Splenocytes were isolated from C57BL/6 and B-hGITR mice (n=3) Flow cytometry analysis of the splenocytes was performed to assess leukocyte subpopulations. A. Representative FACS plots. Single live cells were gated for CD45 population and used for further analysis as indicated here. B. Results of FACS analysis. Percent of T, B, NK, Monocyte, DC and macrophage cells in homozygous B-hGITR mice were similar to those in the C57BL/6 mice, demonstrating that introduction of hGITR in place of its mouse counterpart does not change the overall development, differentiation or distribution of these cell types in spleen.
-
Analysis of T cell subpopulation in B-hGITR mice

-

Analysis of spleen T cell subpopulations by FACS. Splenocytes were isolated from female C57BL/6 and B-hGITR mice (n=3). Flow cytometry analysis of the splenocytes was performed to assess leukocyte subpopulations. A. The proportion of Tcell subpopulation was tested by flow cytometry. B. Results of FACS analysis. Percent of CD8, CD4, and Treg cells in homozygous B-hGITR mice were similar to those in the C57BL/6 mice, demonstrating that introduction of hGITR in place of its mouse counterpart does not change the overall development, differentiation or distribution of these T cell sub types in spleen.
-
Analysis of leukocytes cell subpopulation in B-hGITR mice

-
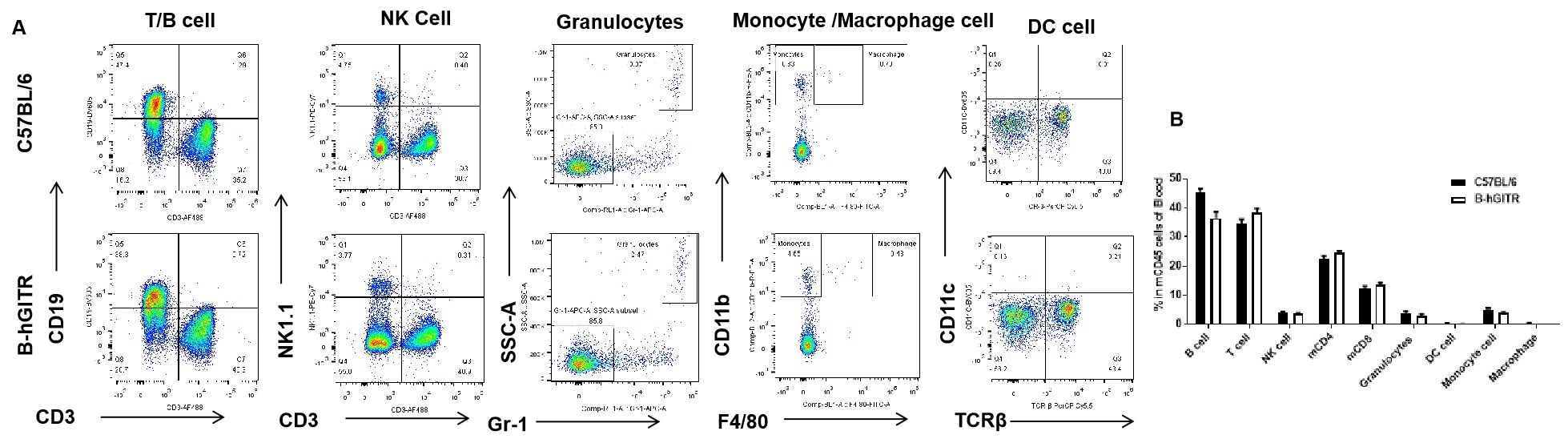
Analysis of blood leukocyte subpopulations by FACS. Blood were isolated from C57BL/6 and B-hGITR mice (n=3) Flow cytometry analysis of the splenocytes was performed to assess leukocyte subpopulations. A. Representative FACS plots. Single live cells were gated for CD45 population and used for further analysis as indicated here. B. Results of FACS analysis. Percent of T, B, NK, Monocyte, DC and macrophage cells in homozygous B-hGITR mice were similar to those in the C57BL/6 mice, demonstrating that introduction of hGITR in place of its mouse counterpart does not change the overall development, differentiation or distribution of these cell types in spleen.
-
Analysis of T cell subpopulation in B-hGITR mice

-
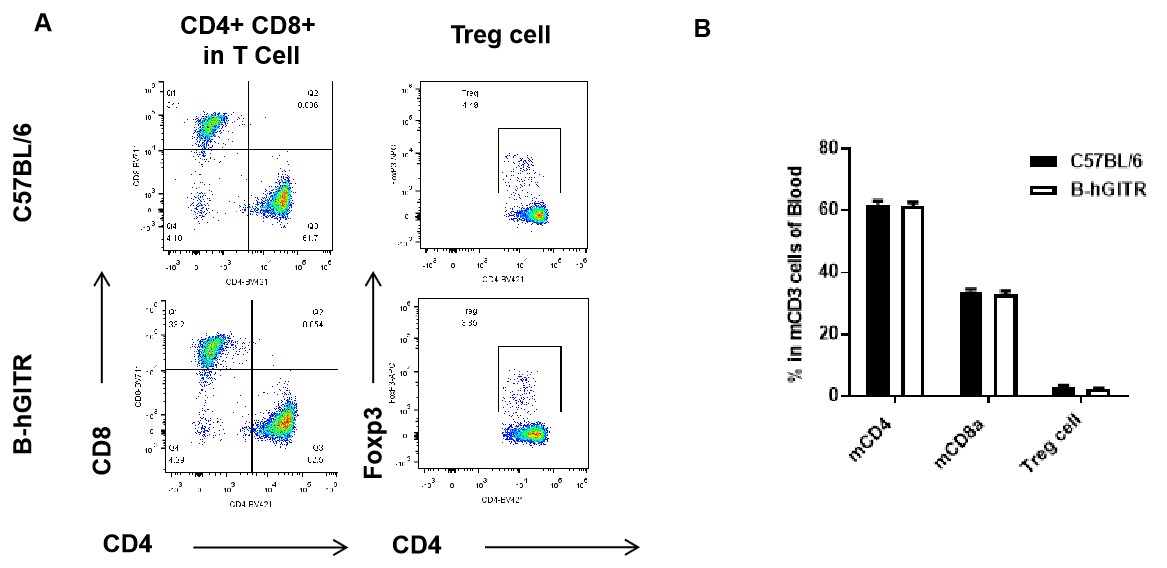
Analysis of blood T cell subpopulations by FACS. Blood were isolated from female C57BL/6 and B-hGITR mice (n=3). Flow cytometry analysis of the splenocytes was performed to assess leukocyte subpopulations. A. The proportion of Tcell subpopulation was tested by flow cytometry. B. Results of FACS analysis. Percent of CD8, CD4, and Treg cells in homozygous B-hGITR mice were similar to those in the C57BL/6 mice, demonstrating that introduction of hGITR in place of its mouse counterpart does not change the overall development, differentiation or distribution of these T cell sub types in spleen.
-
In vivo efficacy of anti-human GITR antibodies

-
In vivo efficacy of anti-human GITR antibodies
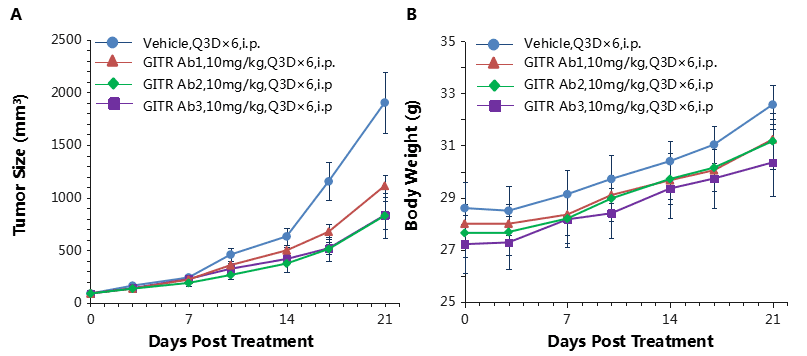
Antitumor activity of anti-human GITR antibodies in B-hGITR mice. (A) Anti-human GITR antibodies inhibited MC38 tumor growth in B-hGITR mice. Murine colon cancer MC38 cells (5ⅹ105) were subcutaneously implanted into heterozygous B-hGITR mice (female, 9 week-old, n=5). Mice were grouped when tumor volume reached approximately 150±50 mm3, at which time they were treated with three anti-human GITR antibodies with doses and schedules indicated in panel (B) Body weight changes during treatment. As shown in panel A, anti-human GITR antibodies were efficacious in controlling tumor growth in B-hGITR mice, demonstrating that the B-hGITR mice provide a powerful preclinical model for in vivo evaluation of anti-human GITR antibodies. Values are expressed as mean ± SEM.
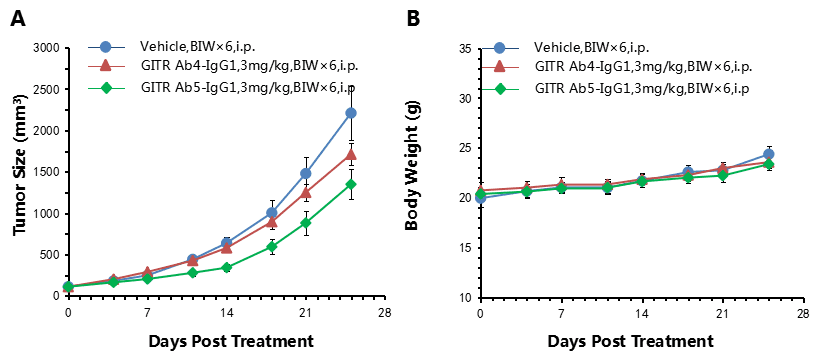
Antitumor activity of anti-human GITR antibodies in B-hGITR mice. (A) Anti-human GITR antibodies inhibited MC38 tumor growth in B-hGITR mice. Murine colon cancer MC38 cells (5ⅹ105) were subcutaneously implanted into homozygote B-hGITR mice (female, 5-8 week-old, n=5). Mice were grouped when tumor volume reached approximately 150±50 mm3, at which time they were treated with two anti-human GITR antibodies with doses and schedules indicated in panel A. (B) Body weight changes during treatment. As shown in panel A, anti-human GITR antibody was efficacious in controlling tumor growth in B-hGITR mice, demonstrating that the B-hGITR mice provide a powerful preclinical model for in vivo evaluation of anti-human GITR antibodies. Values are expressed as mean ± SEM.
-
References

-
- ONCOIMMUNOLOGY 2017, VOL. 6, NO. 3, e1280645 (14 pages) doi: 10.1080/2162402X.2017.1280645
- Cancer Immunol Res; 1(5) November 2013 doi:10.1158/2326-6066.CIR-13-0086


