Basic Information
-
Gene targeting strategy

-
Gene targeting strategy for B-hFcRn mice. The human full-length FCGRT cDNA sequence was inserted in the Fcgrt exon2 of wild-type mice.
-
mRNA expression analysis

-
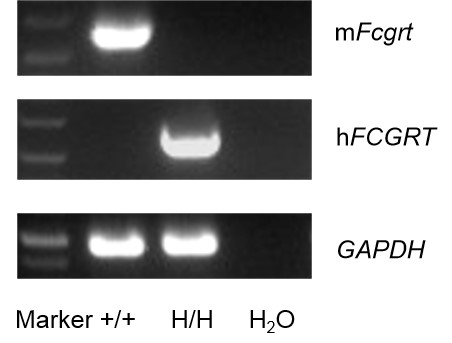
Strain specific analysis of FCGRT gene expression in WT and B-hFcRn mice by RT-PCR. Mouse Fcgrt mRNA was detectable in kidney of wild-type (+/+). Human FCGRT mRNA was detectable only in H/H but not in +/+ mice.
-
Protein expression analysis

-
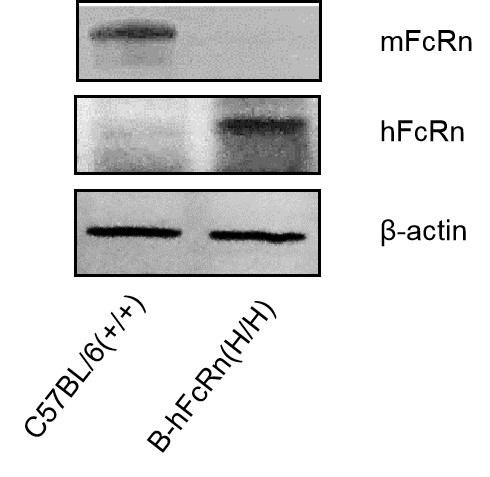
Strain specific analysis of FcRn expression in C57BL/6(+/+) and B-hFcRn mice by western blot. Kidney was collected from C57BL/6(+/+) mice and homozygous B-hFcRn(H/H) mice and analyzed by western blot with species-specific anti-FcRn antibody. Mouse FcRn was detectable in C57BL/6(+/+) mice. Human FcRn was detectable only in B-hFcRn(H/H) but not in C57BL/6(+/+) mice.
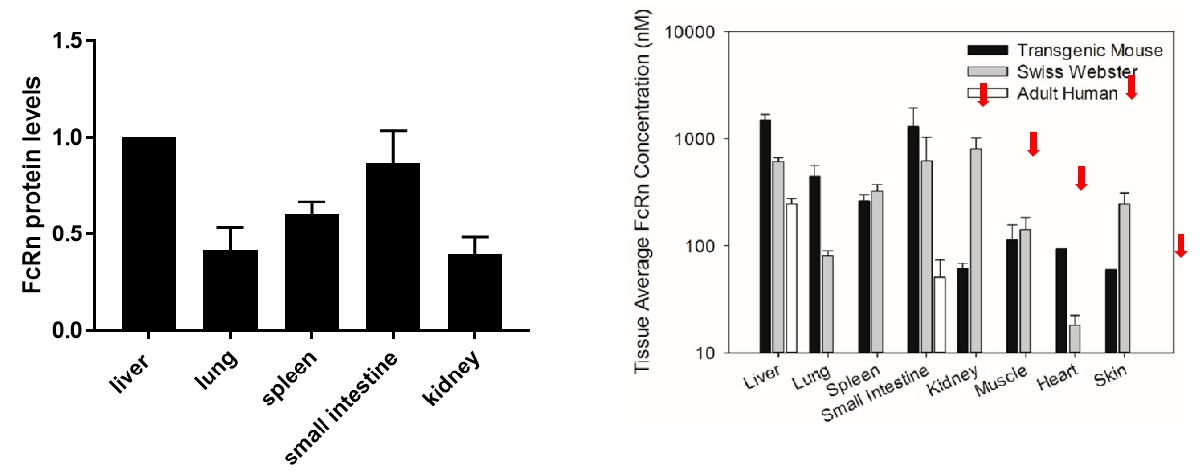
Similar expression pattern between B-hFcRn mice and transgenic mice reported in papers.
-
The YTE mutation prolongs Abs half-life in B-hFcRn mice

-


The serum pharmacokinetics profiles for Ab1 and Ab1-YTE are shown. The effect of YTE in prolonging the half-life of antibodies is only observed in B-hFcRn mice but not in wild-type mice.
-
Serum half-life of commercial antibody drug in B-hFcRn mice

-


- The test drugs are commercial drugs
- The half-life in humans and monkeys of test drugs from research articles
- Blood collection: Continuous
- Abrupt PK changes were excluded in the analyses
- Curve fitting: The last three blood collection points
- Data are shown in mean ± SEM
-
Correlation of half-lives of Abs between human, monkey, B-hFcRn and WT mice

-
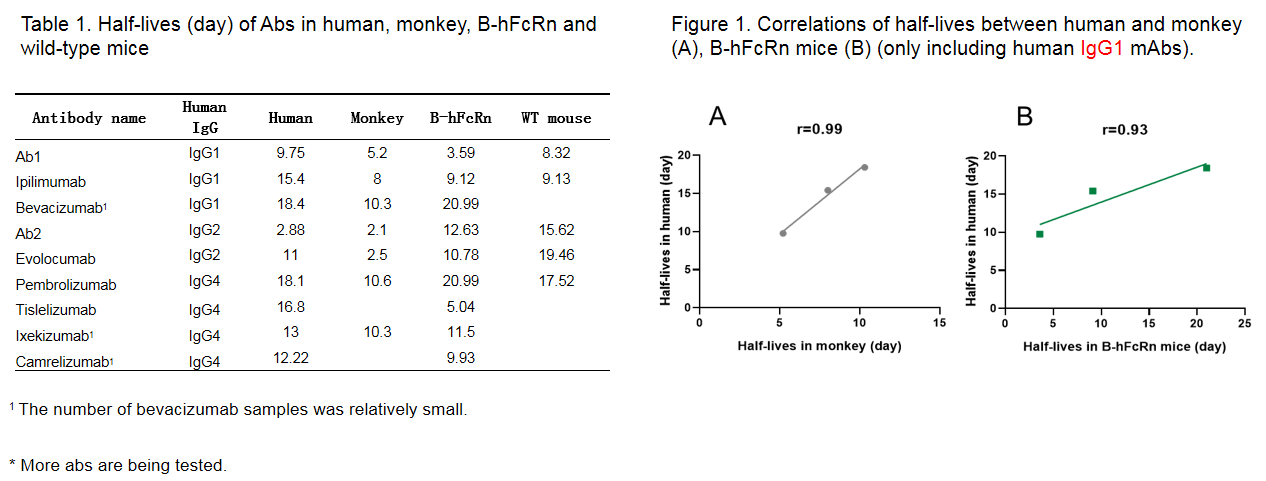
-
References

-
- PMDA. Pembrolizumab. https://www.pmda.go.jp/files/000226881.pdf
- PMDA. Evolocumab. https://www.pmda.go.jp/files/000217925.pdf
- PMDA. Bevacizumab. https://www.pmda.go.jp/files/000224437.pdf
- EMA. Repatha- Product information. https://www.ema.europa.eu/en/documents/product-information/repatha-epar-product-information_en.pdf
- EMA. Yervoy- Product information. https://www.ema.europa.eu/en/documents/product-information/yervoy-epar-product-information_en.pdf
- EMA. Taltz: EPAR- Product information. https://www.ema.europa.eu/en/documents/product-information/taltz-epar-product-information_en.pdf
- Tislelizumab: Desai J, Deva S, Lee JS, Lin CC, Yen CJ, Chao Y, Keam B, Jameson M, Hou MM, Kang YK, Markman B, Lu CH, Rau KM, Lee KH, Horvath L, Friedlander M, Hill A, Sandhu S, Barlow P, Wu CY, Zhang Y, Liang L, Wu J, Paton V, Millward M. Phase IA/IB study of single-agent tislelizumab, an investigational anti-PD-1 antibody, in solid tumors. J Immunother Cancer. 2020 Jun;8(1):e000453.
- Ixekizumab: Liu L, Lu J, Allan BW, Tang Y, Tetreault J, Chow CK, Barmettler B, Nelson J, Bina H, Huang L, Wroblewski VJ, Kikly K. Generation and characterization of ixekizumab, a humanized monoclonal antibody that neutralizes interleukin-17A. J Inflamm Res. 2016 Apr 19;9:39-50.
- Camrelizumab. Zhou L, Wu X, Chi Z, Si L, Sheng X, Kong Y, Mao L, Lian B, Tang B, Yan X, Wang X, Bai X, Li S, Wei X, Li J, Yang Q, Guo J, Cui C. Safety, activity, and pharmacokinetics of camrelizumab in advanced Asian melanoma patients: a phase I study. BMC Cancer. 2022 May 20;22(1):565.
- Bevacizumab. Herbst RS, Johnson DH, Mininberg E, et al. (2005). Phase I/II trial evaluating the anti-vascular endothelial growth factor monoclonal antibody bevacizumab in combination with the HER-1/epidermal growth factor receptor tyrosine kinase inhibitor erlotinib for patients with recurrent non-small-cell lung cancer. J Clin Oncol 23:2544–55.


